当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insight into the Role of an α-Helix Cluster in Protoporphyrinogen IX Oxidase
Biochemistry ( IF 2.9 ) Pub Date : 2024-01-29 , DOI: 10.1021/acs.biochem.3c00508 Baifan Wang 1 , Yiban Wang 1 , Zijuan Zhang 1 , Xin Wen 1 , Zhen Xi 1
Biochemistry ( IF 2.9 ) Pub Date : 2024-01-29 , DOI: 10.1021/acs.biochem.3c00508 Baifan Wang 1 , Yiban Wang 1 , Zijuan Zhang 1 , Xin Wen 1 , Zhen Xi 1
Affiliation
|
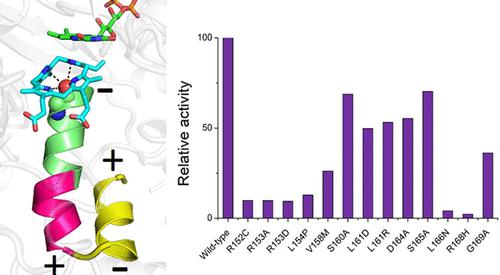
Protoporphyrinogen IX oxidase (PPO) is the last common enzyme in chlorophyll and heme biosynthesis pathways. In humans, point mutations on PPO are responsible for the dominantly inherited disorder disease variegate porphyria (VP). It is found that several VP-causing mutation sites are located on an α-helix cluster (consisting of α-5, α-6, and α-7 helix, named the G169 helix cluster) of human PPO, although these mutation sites are outside the active site of the human PPO. In this work, we investigated the role of the G169 helix cluster via site-directed mutagenesis, enzymatic kinetics, and computational studies. Kinetic studies showed that mutations on the G169 helix cluster affect the activity of PPO. The MD simulation showed that mutations on the G169 helix cluster reduced the activity of PPO by affecting the proper orientation of substrate protoporphyrinogen within the active site of PPO and possibly the dipole moment of the G169 helix cluster. Moreover, the mutation abolished the interaction between the mutated site and other residues, thus affecting the secondary structure and hydrogen bond interactions within the G169 helix cluster. These results indicated that the integrity of the G169 helix cluster is important for the stabilization of protoporphyrinogen within the active site of PPO to facilitate the interaction between protoporphyrinogen and cofactor FAD and provide a proper electrostatic environment for the activity of PPO. Our result provides new insight into understanding the relationship between the structure and function of PPO.
中文翻译:

深入了解 α-螺旋簇在原卟啉原 IX 氧化酶中的作用
原卟啉原 IX 氧化酶 (PPO) 是叶绿素和血红素生物合成途径中最后一种常见的酶。在人类中,PPO 上的点突变导致显性遗传性疾病杂色卟啉症 (VP)。研究发现,人类PPO的一个α螺旋簇(由α-5、α-6和α-7螺旋组成,称为G169螺旋簇)上有几个引起VP的突变位点,尽管这些突变位点是位于人类 PPO 活性位点之外。在这项工作中,我们通过定点诱变、酶动力学和计算研究研究了 G169 螺旋簇的作用。动力学研究表明,G169 螺旋簇上的突变会影响 PPO 的活性。MD模拟表明,G169螺旋簇上的突变通过影响底物原卟啉原在PPO活性位点内的正确方向以及可能影响G169螺旋簇的偶极矩来降低PPO的活性。此外,该突变消除了突变位点与其他残基之间的相互作用,从而影响了G169螺旋簇内的二级结构和氢键相互作用。这些结果表明,G169螺旋簇的完整性对于原卟啉原在PPO活性位点内的稳定非常重要,有利于原卟啉原与辅因子FAD之间的相互作用,并为PPO的活性提供适当的静电环境。我们的结果为理解 PPO 结构和功能之间的关系提供了新的见解。
更新日期:2024-01-29
中文翻译:

深入了解 α-螺旋簇在原卟啉原 IX 氧化酶中的作用
原卟啉原 IX 氧化酶 (PPO) 是叶绿素和血红素生物合成途径中最后一种常见的酶。在人类中,PPO 上的点突变导致显性遗传性疾病杂色卟啉症 (VP)。研究发现,人类PPO的一个α螺旋簇(由α-5、α-6和α-7螺旋组成,称为G169螺旋簇)上有几个引起VP的突变位点,尽管这些突变位点是位于人类 PPO 活性位点之外。在这项工作中,我们通过定点诱变、酶动力学和计算研究研究了 G169 螺旋簇的作用。动力学研究表明,G169 螺旋簇上的突变会影响 PPO 的活性。MD模拟表明,G169螺旋簇上的突变通过影响底物原卟啉原在PPO活性位点内的正确方向以及可能影响G169螺旋簇的偶极矩来降低PPO的活性。此外,该突变消除了突变位点与其他残基之间的相互作用,从而影响了G169螺旋簇内的二级结构和氢键相互作用。这些结果表明,G169螺旋簇的完整性对于原卟啉原在PPO活性位点内的稳定非常重要,有利于原卟啉原与辅因子FAD之间的相互作用,并为PPO的活性提供适当的静电环境。我们的结果为理解 PPO 结构和功能之间的关系提供了新的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号