Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-01-31 , DOI: 10.3762/bjoc.20.18 Anna V Orlova , Nelly N Malysheva , Maria V Panova , Nikita M Podvalnyy , Michael G Medvedev , Leonid O Kononov
Abstract
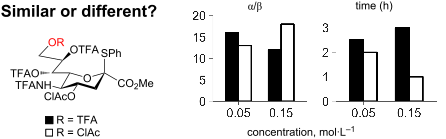
The development of new methods for chemical glycosylation commonly includes comparison of various glycosyl donors. An attempted comparison of chemical properties of two sialic acid-based thioglycoside glycosyl donors, differing only in the substituent at O-9 (trifluoroacetyl vs chloroacetyl), at different concentrations (0.05 and 0.15 mol·L−1) led to mutually excluding conclusions concerning their relative reactivity and selectivity, which prevented us from revealing a possible influence of remote protective groups at O-9 on glycosylation outcome. According to the results of the supramer analysis of the reaction solutions, this issue might be related to the formation of supramers of glycosyl donors differing in structure hence chemical properties. These results seem to imply that comparison of chemical properties of different glycosyl donors may not be as simple and straightforward as it is usually considered.
Beilstein J. Org. Chem. 2024, 20, 181–192. doi:10.3762/bjoc.20.18
中文翻译:

糖基供体的比较:超聚体方法
摘要
化学糖基化新方法的开发通常包括对各种糖基供体的比较。尝试比较两种基于唾液酸的硫代糖苷糖基供体的化学性质,仅在 O-9 处的取代基不同(三氟乙酰基与氯乙酰基),在不同浓度(0.05 和 0.15 mol·L -1)下导致相互排斥的结论它们的相对反应性和选择性,这使我们无法揭示 O-9 上的远程保护基团对糖基化结果的可能影响。根据反应溶液的超聚体分析结果,该问题可能与糖基供体形成的超聚体结构不同、化学性质不同有关。这些结果似乎意味着不同糖基供体化学性质的比较可能并不像通常认为的那么简单和直接。

贝尔斯坦 J. 组织。化学。 2024, 20, 181–192。doi:10.3762/bjoc.20.18



























 京公网安备 11010802027423号
京公网安备 11010802027423号