当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dissecting the Interaction between Cryptochrome and Timeless Reveals Underpinnings of Light-Dependent Recognition
Biochemistry ( IF 2.9 ) Pub Date : 2024-01-31 , DOI: 10.1021/acs.biochem.3c00630 Connor M. Schneps 1 , Robert Dunleavy 1 , Brian R. Crane 1
Biochemistry ( IF 2.9 ) Pub Date : 2024-01-31 , DOI: 10.1021/acs.biochem.3c00630 Connor M. Schneps 1 , Robert Dunleavy 1 , Brian R. Crane 1
Affiliation
|
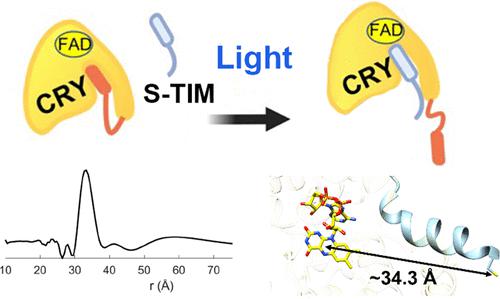
Circadian rhythms are determined by cell-autonomous transcription–translation feedback loops that entrain to environmental stimuli. In the model circadian clock of Drosophila melanogaster, the clock is set by the light-induced degradation of the core oscillator protein timeless (TIM) by the principal light-sensor cryptochrome (CRY). The cryo-EM structure of CRY bound to TIM revealed that within the extensive CRY:TIM interface, the TIM N-terminus binds into the CRY FAD pocket, in which FAD and the associated phosphate-binding loop (PBL) undergo substantial rearrangement. The TIM N-terminus involved in CRY binding varies in isoforms that facilitate the adaptation of flies to different light environments. Herein, we demonstrate, through peptide binding assays and pulsed-dipolar electron spin resonance (ESR) spectroscopy, that the TIM N-terminal peptide alone exhibits light-dependent binding to CRY and that the affinity of the interaction depends on the initiating methionine residue. Extensions to the TIM N-terminus that mimic less light-sensitive variants have substantially reduced interactions with CRY. Substitutions of CRY residues that couple to the flavin rearrangement in the CRY:TIM complex have dramatic effects on CRY light activation. CRY residues Arg237 on α8, Asn253, and Gln254 on the PBL are critical for the release of the CRY autoinhibitory C-terminal tail (CTT) and subsequent TIM binding. These key light-responsive elements of CRY are well conserved throughout Type I cryptochromes of invertebrates but not by cryptochromes of chordates and plants, which likely utilize a distinct light-activation mechanism.
中文翻译:

剖析隐花色素和永恒之间的相互作用揭示了光依赖性识别的基础
昼夜节律是由受到环境刺激的细胞自主转录-翻译反馈环决定的。在果蝇的生物钟模型中,时钟是通过主要光传感器隐花色素(CRY)对核心振荡器蛋白永恒(TIM)的光诱导降解来设定的。CRY 与 TIM 结合的冷冻电镜结构表明,在广泛的 CRY:TIM 界面内,TIM N 末端结合到 CRY FAD 口袋中,其中 FAD 和相关的磷酸盐结合环 (PBL) 发生大量重排。参与 CRY 结合的 TIM N 末端有多种亚型,有助于果蝇适应不同的光环境。在此,我们通过肽结合测定和脉冲偶极电子自旋共振 (ESR) 光谱证明,TIM N 端肽单独表现出与 CRY 的光依赖性结合,并且相互作用的亲和力取决于起始蛋氨酸残基。模仿光敏感度较低的变体的 TIM N 末端延伸大大减少了与 CRY 的相互作用。与 CRY:TIM 复合物中黄素重排耦合的 CRY 残基取代对 CRY 光激活具有显着影响。PBL 上 α8、Asn253 和 Gln254 上的 CRY 残基 Arg237 对于 CRY 自抑制 C 末端尾部 (CTT) 的释放和随后的 TIM 结合至关重要。CRY 的这些关键光响应元件在无脊椎动物的 I 型隐花色素中得到了很好的保守,但脊索动物和植物的隐花色素却不然,后者可能利用了独特的光激活机制。
更新日期:2024-01-31
中文翻译:

剖析隐花色素和永恒之间的相互作用揭示了光依赖性识别的基础
昼夜节律是由受到环境刺激的细胞自主转录-翻译反馈环决定的。在果蝇的生物钟模型中,时钟是通过主要光传感器隐花色素(CRY)对核心振荡器蛋白永恒(TIM)的光诱导降解来设定的。CRY 与 TIM 结合的冷冻电镜结构表明,在广泛的 CRY:TIM 界面内,TIM N 末端结合到 CRY FAD 口袋中,其中 FAD 和相关的磷酸盐结合环 (PBL) 发生大量重排。参与 CRY 结合的 TIM N 末端有多种亚型,有助于果蝇适应不同的光环境。在此,我们通过肽结合测定和脉冲偶极电子自旋共振 (ESR) 光谱证明,TIM N 端肽单独表现出与 CRY 的光依赖性结合,并且相互作用的亲和力取决于起始蛋氨酸残基。模仿光敏感度较低的变体的 TIM N 末端延伸大大减少了与 CRY 的相互作用。与 CRY:TIM 复合物中黄素重排耦合的 CRY 残基取代对 CRY 光激活具有显着影响。PBL 上 α8、Asn253 和 Gln254 上的 CRY 残基 Arg237 对于 CRY 自抑制 C 末端尾部 (CTT) 的释放和随后的 TIM 结合至关重要。CRY 的这些关键光响应元件在无脊椎动物的 I 型隐花色素中得到了很好的保守,但脊索动物和植物的隐花色素却不然,后者可能利用了独特的光激活机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号