当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterization of an Acinetobacter baumannii Monofunctional Phosphomethylpyrimidine Kinase That Is Inhibited by Pyridoxal Phosphate
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-02 , DOI: 10.1021/acs.biochem.3c00640 Humberto De Vitto 1 , Kafi K. J. Belfon 2 , Nandini Sharma 1 , Sarah Toay 3 , Jan Abendroth 4, 5 , David. M. Dranow 4, 5 , Christine M. Lukacs 4, 5 , Ryan Choi 5 , Hannah S. Udell 5 , Sydney Willis 6 , George Barrera 7 , Olive Beyer 8 , Teng Da Li 2 , Katherine A. Hicks 9 , Andrew T. Torelli 10 , Jarrod B. French 1
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-02 , DOI: 10.1021/acs.biochem.3c00640 Humberto De Vitto 1 , Kafi K. J. Belfon 2 , Nandini Sharma 1 , Sarah Toay 3 , Jan Abendroth 4, 5 , David. M. Dranow 4, 5 , Christine M. Lukacs 4, 5 , Ryan Choi 5 , Hannah S. Udell 5 , Sydney Willis 6 , George Barrera 7 , Olive Beyer 8 , Teng Da Li 2 , Katherine A. Hicks 9 , Andrew T. Torelli 10 , Jarrod B. French 1
Affiliation
|
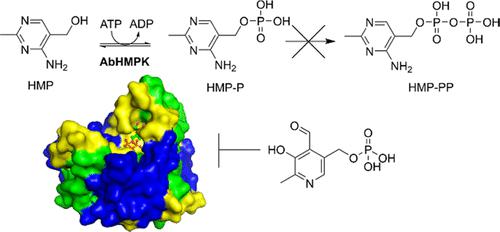
Thiamin and its phosphate derivatives are ubiquitous molecules involved as essential cofactors in many cellular processes. The de novo biosynthesis of thiamin employs the parallel synthesis of 4-methyl-5-(2-hydroxyethyl)thiazole (THZ-P) and 4-amino-2-methyl-5(diphosphooxymethyl) pyrimidine (HMP) pyrophosphate (HMP-PP), which are coupled to generate thiamin phosphate. Most organisms that can biosynthesize thiamin employ a kinase (HMPK or ThiD) to generate HMP-PP. In nearly all cases, this enzyme is bifunctional and can also salvage free HMP, producing HMP-P, the monophosphate precursor of HMP-PP. Here we present high-resolution crystal structures of an HMPK from Acinetobacter baumannii (AbHMPK), both unliganded and with pyridoxal 5-phosphate (PLP) noncovalently bound. Despite the similarity between HMPK and pyridoxal kinase enzymes, our kinetics analysis indicates that AbHMPK accepts HMP exclusively as a substrate and cannot turn over pyridoxal, pyridoxamine, or pyridoxine nor does it display phosphatase activity. PLP does, however, act as a weak inhibitor of AbHMPK with an IC50 of 768 μM. Surprisingly, unlike other HMPKs, AbHMPK catalyzes only the phosphorylation of HMP and does not generate the diphosphate HMP-PP. This suggests that an additional kinase is present in A. baumannii, or an alternative mechanism is in operation to complete the biosynthesis of thiamin.
中文翻译:

受磷酸吡哆醛抑制的鲍曼不动杆菌单功能磷酸甲基嘧啶激酶的表征
硫胺素及其磷酸盐衍生物是普遍存在的分子,作为许多细胞过程中必需的辅助因子。硫胺素的从头生物合成采用 4-甲基-5-(2-羟乙基)噻唑 (THZ-P) 和 4-氨基-2-甲基-5(二磷氧基甲基)嘧啶 (HMP) 焦磷酸盐 (HMP-PP) 的平行合成),它们偶联生成磷酸硫胺素。大多数能够生物合成硫胺素的生物体利用激酶(HMPK 或 ThiD)来生成 HMP-PP。几乎在所有情况下,这种酶都是双功能的,还可以回收游离的 HMP,产生 HMP-P,即 HMP-PP 的单磷酸盐前体。在这里,我们展示了来自鲍曼不动杆菌(AbHMPK)的 HMPK 的高分辨率晶体结构,包括未配体的和与 5-磷酸吡哆醛 (PLP) 非共价结合的。尽管 HMPK 和吡哆醛激酶之间存在相似性,但我们的动力学分析表明 AbHMPK 只接受 HMP 作为底物,不能转化吡哆醛、吡哆胺或吡哆醇,也不显示磷酸酶活性。然而,PLP 确实是 AbHMPK 的弱抑制剂,IC 50为 768 μM。令人惊讶的是,与其他 HMPK 不同,AbHMPK 只催化 HMP 的磷酸化,不会生成二磷酸 HMP-PP。这表明鲍曼不动杆菌中存在额外的激酶,或者正在运行替代机制来完成硫胺素的生物合成。
更新日期:2024-02-02
中文翻译:

受磷酸吡哆醛抑制的鲍曼不动杆菌单功能磷酸甲基嘧啶激酶的表征
硫胺素及其磷酸盐衍生物是普遍存在的分子,作为许多细胞过程中必需的辅助因子。硫胺素的从头生物合成采用 4-甲基-5-(2-羟乙基)噻唑 (THZ-P) 和 4-氨基-2-甲基-5(二磷氧基甲基)嘧啶 (HMP) 焦磷酸盐 (HMP-PP) 的平行合成),它们偶联生成磷酸硫胺素。大多数能够生物合成硫胺素的生物体利用激酶(HMPK 或 ThiD)来生成 HMP-PP。几乎在所有情况下,这种酶都是双功能的,还可以回收游离的 HMP,产生 HMP-P,即 HMP-PP 的单磷酸盐前体。在这里,我们展示了来自鲍曼不动杆菌(AbHMPK)的 HMPK 的高分辨率晶体结构,包括未配体的和与 5-磷酸吡哆醛 (PLP) 非共价结合的。尽管 HMPK 和吡哆醛激酶之间存在相似性,但我们的动力学分析表明 AbHMPK 只接受 HMP 作为底物,不能转化吡哆醛、吡哆胺或吡哆醇,也不显示磷酸酶活性。然而,PLP 确实是 AbHMPK 的弱抑制剂,IC 50为 768 μM。令人惊讶的是,与其他 HMPK 不同,AbHMPK 只催化 HMP 的磷酸化,不会生成二磷酸 HMP-PP。这表明鲍曼不动杆菌中存在额外的激酶,或者正在运行替代机制来完成硫胺素的生物合成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号