当前位置:
X-MOL 学术
›
Comput. Struct. Biotechnol. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Prognostic iron-metabolism signature robustly stratifies single-cell characteristics of hepatocellular carcinoma
Computational and Structural Biotechnology Journal ( IF 6 ) Pub Date : 2024-02-01 , DOI: 10.1016/j.csbj.2024.01.022 Zhipeng Zhu , Huang Cao , Hongyu Yan , Hanzhi Liu , Zaifa Hong , Anran Sun , Tong Liu , Fengbiao Mao
Computational and Structural Biotechnology Journal ( IF 6 ) Pub Date : 2024-02-01 , DOI: 10.1016/j.csbj.2024.01.022 Zhipeng Zhu , Huang Cao , Hongyu Yan , Hanzhi Liu , Zaifa Hong , Anran Sun , Tong Liu , Fengbiao Mao
|
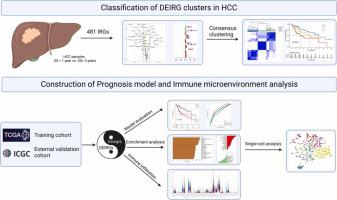
Cancer immunotherapy has shown to be a promising method in treating hepatocellular carcinoma (HCC), but suboptimal responses in patients are attributed to cellular and molecular heterogeneity. Iron metabolism-related genes (IRGs) are important in maintaining immune system homeostasis and have the potential to help develop new strategies for HCC treatment. Herein, we constructed and validated the iron-metabolism gene prognostic index (IPX) using univariate Cox proportional hazards regression and LASSO Cox regression analysis, successfully categorizing HCC patients into two groups with distinct survival risks. Then, we performed single-sample gene set enrichment analysis, weighted correlation network analysis, gene ontology enrichment analysis, cellular lineage analysis, and SCENIC analysis to reveal the key determinants underlying the ability of this model based on bulk and single-cell transcriptomic data. We identified several driver transcription factors specifically activated in specific malignant cell sub-populations to contribute to the adverse survival outcomes in the IPX-high subgroup. Within the tumor microenvironment (TME), T cells displayed significant diversity in their cellular characteristics and experienced changes in their developmental paths within distinct clusters identified by IPX. Interestingly, the proportion of Treg cells was increased in the high-risk group compared with the low-risk group. These results suggest that iron-metabolism could be involved in reshaping the TME, thereby disrupting the cell cycle of immune cells. This study utilized IRGs to construct a novel and reliable model, which can be used to assess the prognosis of patients with HCC and further clarify the molecular mechanisms of IRGs in HCC at single-cell resolution.
中文翻译:

预后铁代谢特征对肝细胞癌的单细胞特征进行了强有力的分层
癌症免疫疗法已被证明是治疗肝细胞癌(HCC)的一种有前途的方法,但患者的反应欠佳归因于细胞和分子异质性。铁代谢相关基因 (IRG) 对于维持免疫系统稳态很重要,并有可能帮助制定 HCC 治疗的新策略。在此,我们使用单变量 Cox 比例风险回归和 LASSO Cox 回归分析构建并验证了铁代谢基因预后指数 (IPX),成功将 HCC 患者分为具有不同生存风险的两组。然后,我们进行了单样本基因集富集分析、加权相关网络分析、基因本体富集分析、细胞谱系分析和SCENIC分析,以基于大量和单细胞转录组数据揭示该模型能力的关键决定因素。我们确定了在特定恶性细胞亚群中特异性激活的几种驱动转录因子,这些因子导致 IPX 高亚群的不良生存结果。在肿瘤微环境 (TME) 中,T 细胞表现出显着的细胞特征多样性,并在 IPX 识别的不同簇内经历了发育路径的变化。有趣的是,与低风险组相比,高风险组的Treg细胞比例有所增加。这些结果表明铁代谢可能参与重塑 TME,从而破坏免疫细胞的细胞周期。本研究利用IRGs构建了一种新颖且可靠的模型,可用于评估HCC患者的预后,并在单细胞分辨率下进一步阐明IRGs在HCC中的分子机制。
更新日期:2024-02-01
中文翻译:

预后铁代谢特征对肝细胞癌的单细胞特征进行了强有力的分层
癌症免疫疗法已被证明是治疗肝细胞癌(HCC)的一种有前途的方法,但患者的反应欠佳归因于细胞和分子异质性。铁代谢相关基因 (IRG) 对于维持免疫系统稳态很重要,并有可能帮助制定 HCC 治疗的新策略。在此,我们使用单变量 Cox 比例风险回归和 LASSO Cox 回归分析构建并验证了铁代谢基因预后指数 (IPX),成功将 HCC 患者分为具有不同生存风险的两组。然后,我们进行了单样本基因集富集分析、加权相关网络分析、基因本体富集分析、细胞谱系分析和SCENIC分析,以基于大量和单细胞转录组数据揭示该模型能力的关键决定因素。我们确定了在特定恶性细胞亚群中特异性激活的几种驱动转录因子,这些因子导致 IPX 高亚群的不良生存结果。在肿瘤微环境 (TME) 中,T 细胞表现出显着的细胞特征多样性,并在 IPX 识别的不同簇内经历了发育路径的变化。有趣的是,与低风险组相比,高风险组的Treg细胞比例有所增加。这些结果表明铁代谢可能参与重塑 TME,从而破坏免疫细胞的细胞周期。本研究利用IRGs构建了一种新颖且可靠的模型,可用于评估HCC患者的预后,并在单细胞分辨率下进一步阐明IRGs在HCC中的分子机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号