当前位置:
X-MOL 学术
›
Eur. J. Nerosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Estrogen-mediated coupling via gap junctions in the suprachiasmatic nucleus
European Journal of Neroscience ( IF 3.4 ) Pub Date : 2024-02-07 , DOI: 10.1111/ejn.16270 Lina Schlaeger 1 , Iwona Olejniczak 1 , Marianne Lehmann 1 , Cosima Xenia Schmidt 1 , Mariana Astiz 1, 2, 3 , Henrik Oster 1 , Violetta Pilorz 1
European Journal of Neroscience ( IF 3.4 ) Pub Date : 2024-02-07 , DOI: 10.1111/ejn.16270 Lina Schlaeger 1 , Iwona Olejniczak 1 , Marianne Lehmann 1 , Cosima Xenia Schmidt 1 , Mariana Astiz 1, 2, 3 , Henrik Oster 1 , Violetta Pilorz 1
Affiliation
|
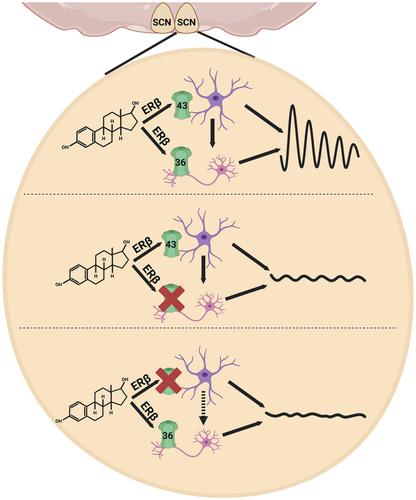
The circadian clock orchestrates many physiological and behavioural rhythms in mammals with 24-h periodicity, through a hierarchical organisation, with the central clock located in the suprachiasmatic nucleus (SCN) in the hypothalamus. The circuits of the SCN generate circadian rhythms with precision, relying on intrinsic coupling mechanisms, for example, neurotransmitters like arginine vasopressin (AVP), vasoactive intestinal peptide (VIP), neuronal gamma-aminobutyric acid (GABA) signalling and astrocytes connected by gap junctions composed of connexins (Cx). In female rodents, the presence of estrogen receptors (ERs) in the dorsal SCN suggests an influence of estrogen (E2) on the circuit timekeeping that could regulate circadian rhythm and coupling. To investigate this, we used SCN explants together with hypothalamic neurons and astrocytes. First, we showed that E2 stabilised the circadian amplitude in the SCN when rAVPs (receptor-associated vasopressin peptides) were inhibited. However, the phase delay induced by VIPAC2 (VIP receptors) inhibition remained unaffected by E2. We then showed that E2 exerted its effects in the SCN via ERβ (estrogen receptor beta), resulting in increased expression of Cx36 and Cx43. Notably, specific inhibition of both connexins resulted in a significant reduction in circadian amplitude within the SCN. Remarkably, E2 restored the period with inhibited Cx36 but not with Cx43 inhibition. This implies that the network between astrocytes and neurons, responsible for coupling in the SCN, can be reinforced through E2. In conclusion, these findings provide new insights into how E2 regulates circadian rhythms ex vivo in an ERβ-dependent manner, underscoring its crucial role in fortifying the SCN's rhythm.
中文翻译:

雌激素通过视交叉上核间隙连接介导的耦合
生物钟通过分层组织以 24 小时为周期协调哺乳动物的许多生理和行为节律,其中央时钟位于下丘脑的视交叉上核 (SCN)。 SCN 回路依靠内在耦合机制精确产生昼夜节律,例如精氨酸加压素 (AVP)、血管活性肠肽 (VIP)、神经元 γ-氨基丁酸 (GABA) 信号传导和通过间隙连接连接的星形胶质细胞等神经递质由连接蛋白(Cx)组成。在雌性啮齿类动物中,背侧 SCN 中雌激素受体 (ER) 的存在表明雌激素 (E2) 对调节昼夜节律和耦合的电路计时有影响。为了研究这一点,我们将 SCN 外植体与下丘脑神经元和星形胶质细胞一起使用。首先,我们发现,当 rAVP(受体相关加压素肽)受到抑制时,E2 可以稳定 SCN 的昼夜节律振幅。然而,抑制 VIPAC2(VIP 受体)引起的相位延迟仍然不受 E2 的影响。然后我们发现 E2 通过 ERβ(雌激素受体 β)在 SCN 中发挥作用,导致Cx36和Cx43表达增加。值得注意的是,两种连接蛋白的特异性抑制导致 SCN 内昼夜节律振幅显着降低。值得注意的是,E2 可以恢复抑制 Cx36 的周期,但不能恢复抑制 Cx43 的周期。这意味着星形胶质细胞和神经元之间负责 SCN 耦合的网络可以通过 E2 得到加强。总之,这些发现为 E2 如何以 ERβ 依赖性方式离体调节昼夜节律提供了新的见解,强调了其在强化 SCN 节律中的关键作用。
更新日期:2024-02-07
中文翻译:

雌激素通过视交叉上核间隙连接介导的耦合
生物钟通过分层组织以 24 小时为周期协调哺乳动物的许多生理和行为节律,其中央时钟位于下丘脑的视交叉上核 (SCN)。 SCN 回路依靠内在耦合机制精确产生昼夜节律,例如精氨酸加压素 (AVP)、血管活性肠肽 (VIP)、神经元 γ-氨基丁酸 (GABA) 信号传导和通过间隙连接连接的星形胶质细胞等神经递质由连接蛋白(Cx)组成。在雌性啮齿类动物中,背侧 SCN 中雌激素受体 (ER) 的存在表明雌激素 (E2) 对调节昼夜节律和耦合的电路计时有影响。为了研究这一点,我们将 SCN 外植体与下丘脑神经元和星形胶质细胞一起使用。首先,我们发现,当 rAVP(受体相关加压素肽)受到抑制时,E2 可以稳定 SCN 的昼夜节律振幅。然而,抑制 VIPAC2(VIP 受体)引起的相位延迟仍然不受 E2 的影响。然后我们发现 E2 通过 ERβ(雌激素受体 β)在 SCN 中发挥作用,导致Cx36和Cx43表达增加。值得注意的是,两种连接蛋白的特异性抑制导致 SCN 内昼夜节律振幅显着降低。值得注意的是,E2 可以恢复抑制 Cx36 的周期,但不能恢复抑制 Cx43 的周期。这意味着星形胶质细胞和神经元之间负责 SCN 耦合的网络可以通过 E2 得到加强。总之,这些发现为 E2 如何以 ERβ 依赖性方式离体调节昼夜节律提供了新的见解,强调了其在强化 SCN 节律中的关键作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号