当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility and Solution Thermodynamics of 3,4-Bis(4′-aminofurazan-3′-yl)furoxan in Pure Solvents and Binary Solvent Mixtures
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-02-13 , DOI: 10.1021/acs.jced.3c00674 Wanyu Gao 1 , Yuan Gao 1 , Ziping Yin 1 , Yanfei Sun 1 , Yu Zhang 1 , Longyi Zhu 1 , Jun Luo 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-02-13 , DOI: 10.1021/acs.jced.3c00674 Wanyu Gao 1 , Yuan Gao 1 , Ziping Yin 1 , Yanfei Sun 1 , Yu Zhang 1 , Longyi Zhu 1 , Jun Luo 1
Affiliation
|
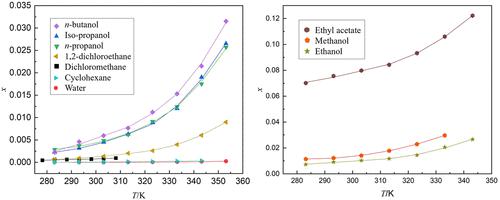
The solubility of 3,4-bis(4′-aminofurazan-3′-yl)furoxan (BAFF) was determined in ten pure solvents (dichloromethane, 1,2-dichloroethane, cyclohexane, water, methanol, ethanol, n-propanol, isopropanol, n-butanol, and ethyl acetate) as well as three binary solvent mixtures (methanol/water, ethanol/water, ethyl acetate/1,2-dichloroethane) over various temperature ranges (283.15 to 353.15 K). The measurements were conducted by using a static analytical method under atmospheric pressure. During the temperature ranges investigated, it was observed that the solubility of BAFF increased with the increase in the temperature in all selected solvents. Among the ten pure solvents, ethyl acetate has the highest solubility, while methanol, ethanol, n-butanol, and isopropanol have moderate solubilities. Water, 1,2-dichloroethane, and dichloromethane have relatively low solubilities, while in the binary solvent systems, the solubility of BAFF decreased with increasing the mole fraction of antisolvent (water or 1,2-dichloroethane) at given temperature. Moreover, the experimental solubility data in pure solvents were correlated using the modified Apelblat equation, the λh equation, and two local composition models (Wilson model and NRTL model). Additionally, the modified Apelblat equation, CNIBS/R-K model, Jouyban-Acree model, and multicomponent NRTL model were successfully employed to correlate the experimental solubility in mixed solvent systems. Furthermore, based on the experimental data and the NRTL model, the dissolution thermodynamic properties of BAFF in both pure and binary solvents were calculated and analyzed. These properties include the dissolution Gibbs energy, enthalpy, and entropy. These data indicate that the dissolution process of BAFF is endothermic and spontaneous.
中文翻译:

3,4-双(4′-氨基呋喃-3′-基)呋喃嗪在纯溶剂和二元溶剂混合物中的溶解度和溶液热力学
测定 3,4-双(4'-氨基呋喃-3'-基)呋喃氧烷 (BAFF) 在 10 种纯溶剂(二氯甲烷、1,2-二氯乙烷、环己烷、水、甲醇、乙醇、正丙醇、不同温度范围(283.15 至 353.15 K)的三种二元溶剂混合物(甲醇/水、乙醇/水、乙酸乙酯/1,2-二氯乙烷)。测量是在大气压下使用静态分析方法进行的。在研究的温度范围内,观察到 BAFF 在所有选定溶剂中的溶解度随着温度的升高而增加。十种纯溶剂中,乙酸乙酯的溶解度最高,甲醇、乙醇、正丁醇、异丙醇的溶解度中等。水、1,2-二氯乙烷和二氯甲烷的溶解度相对较低,而在二元溶剂体系中,在给定温度下,BAFF的溶解度随着反溶剂(水或1,2-二氯乙烷)摩尔分数的增加而降低。此外,使用修正的 Apelblat 方程、λ h方程和两个局部组成模型(Wilson 模型和 NRTL 模型)将纯溶剂中的实验溶解度数据关联起来。此外,改进的 Apelblat 方程、CNIBS/RK 模型、Jouyban-Acree 模型和多组分 NRTL 模型已成功用于关联混合溶剂体系中的实验溶解度。此外,基于实验数据和NRTL模型,计算并分析了BAFF在纯溶剂和二元溶剂中的溶解热力学性质。这些性质包括溶解吉布斯能、焓和熵。这些数据表明 BAFF 的溶解过程是吸热且自发的。
更新日期:2024-02-13
中文翻译:

3,4-双(4′-氨基呋喃-3′-基)呋喃嗪在纯溶剂和二元溶剂混合物中的溶解度和溶液热力学
测定 3,4-双(4'-氨基呋喃-3'-基)呋喃氧烷 (BAFF) 在 10 种纯溶剂(二氯甲烷、1,2-二氯乙烷、环己烷、水、甲醇、乙醇、正丙醇、不同温度范围(283.15 至 353.15 K)的三种二元溶剂混合物(甲醇/水、乙醇/水、乙酸乙酯/1,2-二氯乙烷)。测量是在大气压下使用静态分析方法进行的。在研究的温度范围内,观察到 BAFF 在所有选定溶剂中的溶解度随着温度的升高而增加。十种纯溶剂中,乙酸乙酯的溶解度最高,甲醇、乙醇、正丁醇、异丙醇的溶解度中等。水、1,2-二氯乙烷和二氯甲烷的溶解度相对较低,而在二元溶剂体系中,在给定温度下,BAFF的溶解度随着反溶剂(水或1,2-二氯乙烷)摩尔分数的增加而降低。此外,使用修正的 Apelblat 方程、λ h方程和两个局部组成模型(Wilson 模型和 NRTL 模型)将纯溶剂中的实验溶解度数据关联起来。此外,改进的 Apelblat 方程、CNIBS/RK 模型、Jouyban-Acree 模型和多组分 NRTL 模型已成功用于关联混合溶剂体系中的实验溶解度。此外,基于实验数据和NRTL模型,计算并分析了BAFF在纯溶剂和二元溶剂中的溶解热力学性质。这些性质包括溶解吉布斯能、焓和熵。这些数据表明 BAFF 的溶解过程是吸热且自发的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号