Biochemistry (Moscow) ( IF 2.8 ) Pub Date : 2024-02-14 , DOI: 10.1134/s0006297924010073 Anastasia M. Kochurova , Evgenia A. Beldiia , Victoria V. Nefedova , Natalia S. Ryabkova , Daria S. Yampolskaya , Alexander M. Matyushenko , Sergey Y. Bershitsky , Galina V. Kopylova , Daniil V. Shchepkin
|
Abstract
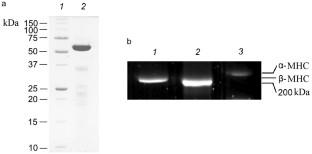
Cardiac myosin binding protein C (cMyBP-C) is one of the essential control components of the myosin cross-bridge cycle. The C-terminal part of cMyBP-C is located on the surface of the thick filament, and its N-terminal part interacts with actin, myosin, and tropomyosin, affecting both kinetics of the ATP hydrolysis cycle and lifetime of the cross-bridge, as well as calcium regulation of the actin–myosin interaction, thereby modulating contractile function of myocardium. The role of cMyBP-C in atrial contraction has not been practically studied. We examined effect of the N-terminal C0-C1-m-C2 (C0-C2) fragment of cMyBP-C on actin–myosin interaction using ventricular and atrial myosin in an in vitro motility assay. The C0-C2 fragment of cMyBP-C significantly reduced the maximum sliding velocity of thin filaments on both myosin isoforms and increased the calcium sensitivity of the actin–myosin interaction. The C0-C2 fragment had different effects on the kinetics of ATP and ADP exchange, increasing the affinity of ventricular myosin for ADP and decreasing the affinity of atrial myosin. The effect of the C0-C2 fragment on the activation of the thin filament depended on the myosin isoforms. Atrial myosin activates the thin filament less than ventricular myosin, and the C0-C2 fragment makes these differences in the myosin isoforms more pronounced.
中文翻译:

心肌肌球蛋白结合蛋白 C 的 N 端片段调节心房和心室细丝激活的协同机制
摘要
心肌肌球蛋白结合蛋白 C (cMyBP-C) 是肌球蛋白跨桥循环的重要控制成分之一。 cMyBP-C的C端部分位于粗丝的表面,其N端部分与肌动蛋白、肌球蛋白和原肌球蛋白相互作用,影响ATP水解循环的动力学和跨桥的寿命,以及肌动蛋白-肌球蛋白相互作用的钙调节,从而调节心肌的收缩功能。 cMyBP-C 在心房收缩中的作用尚未得到实际研究。我们在体外运动测定中使用心室和心房肌球蛋白检查了 cMyBP-C 的 N 末端 C0-C1-m-C2 (C0-C2) 片段对肌动蛋白 - 肌球蛋白相互作用的影响。 cMyBP-C 的 C0-C2 片段显着降低了两种肌球蛋白异构体上细丝的最大滑动速度,并增加了肌动蛋白-肌球蛋白相互作用的钙敏感性。 C0-C2片段对ATP和ADP交换动力学有不同的影响,增加心室肌球蛋白对ADP的亲和力,降低心房肌球蛋白的亲和力。 C0-C2 片段对细丝激活的影响取决于肌球蛋白亚型。心房肌球蛋白对细丝的激活程度低于心室肌球蛋白,并且 C0-C2 片段使肌球蛋白亚型的这些差异更加明显。



























 京公网安备 11010802027423号
京公网安备 11010802027423号