当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biological Evaluation of Antibody-Drug Conjugates Produced by Tag-Free Lipoate Ligase A Modification
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-13 , DOI: 10.1021/acs.biochem.3c00513 Shunsuke Yamazaki 1 , Kenichiro Ito 1 , Tsubasa Aoki 1 , Naoko Arashida 1 , Tomohiro Watanabe 1 , Tomohiro Fujii 1 , Yutaka Matsuda 1
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-13 , DOI: 10.1021/acs.biochem.3c00513 Shunsuke Yamazaki 1 , Kenichiro Ito 1 , Tsubasa Aoki 1 , Naoko Arashida 1 , Tomohiro Watanabe 1 , Tomohiro Fujii 1 , Yutaka Matsuda 1
Affiliation
|
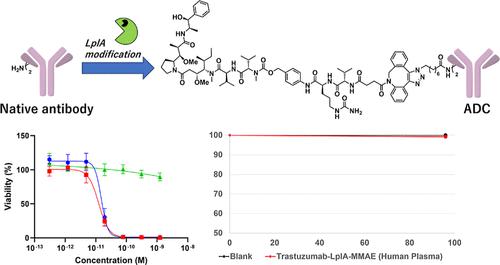
The concept of tag-free protein modification has attracted considerable interest in chemical biology because of its flexible and straightforward reaction process. In 2021, a groundbreaking approach using lipoate ligase A (LplA) for tag-free enzymatic modification of antibodies was unveiled, demonstrating its potential for the generation of precise antibody conjugates. In this study, to further explore LplA-mediated antibody-drug conjugate (ADC) synthesis, we performed initial biological evaluations of ADCs synthesized using LplA. Using the anti-HER2 antibody trastuzumab, we introduced octanoic acid azide using LplA and subsequently obtained an ADC using click chemistry with the drug DBCO-VC-PAB-MMAE. The bioactivity of the synthesized anti-HER2-ADC was evaluated using HER2-positive SKBR-3 and HER2-negative MCF7 cells. Its toxicity and selectivity were found to be comparable to those of the FDA-approved Kadcyla. In addition, a stability study involving rat and human plasma demonstrated the stability of the LplA-mediated ADC. Additionally, the affinity for the neonatal Fc receptor (FcRn) was retained after conjugation. These preliminary in vitro evaluations suggested that LplA-derived ADCs can have considerable pharmaceutical potential. Our results can set the stage for further in vivo evaluations and safety assessments. We suggest that the integration of tag-free LplA methods into the production of ADCs can offer a novel and promising approach for biopharmaceutical manufacturing.
中文翻译:

无标签脂酸连接酶 A 修饰产生的抗体-药物缀合物的生物学评价
无标签蛋白质修饰的概念因其灵活且直接的反应过程而引起了化学生物学的极大兴趣。2021 年,推出了一种使用硫辛酸连接酶 A (LplA) 对抗体进行无标签酶促修饰的突破性方法,证明了其生成精确抗体缀合物的潜力。在本研究中,为了进一步探索 LplA 介导的抗体药物偶联物 (ADC) 合成,我们对使用 LplA 合成的 ADC 进行了初步生物学评估。使用抗 HER2 抗体曲妥珠单抗,我们使用 LplA 引入辛酸叠氮化物,随后使用药物 DBCO-VC-PAB-MMAE 使用点击化学获得 ADC。使用 HER2 阳性 SKBR-3 和 HER2 阴性 MCF7 细胞评估合成的抗 HER2-ADC 的生物活性。其毒性和选择性与 FDA 批准的 Kadcyla 相当。此外,一项涉及大鼠和人血浆的稳定性研究证明了 LplA 介导的 ADC 的稳定性。此外,接合后保留了对新生儿 Fc 受体 (FcRn) 的亲和力。这些初步体外评估表明,LplA 衍生的 ADC 具有相当大的药物潜力。我们的结果可以为进一步的体内评估和安全性评估奠定基础。我们建议将无标签 LplA 方法整合到 ADC 的生产中可以为生物制药制造提供一种新颖且有前景的方法。
更新日期:2024-02-13
中文翻译:

无标签脂酸连接酶 A 修饰产生的抗体-药物缀合物的生物学评价
无标签蛋白质修饰的概念因其灵活且直接的反应过程而引起了化学生物学的极大兴趣。2021 年,推出了一种使用硫辛酸连接酶 A (LplA) 对抗体进行无标签酶促修饰的突破性方法,证明了其生成精确抗体缀合物的潜力。在本研究中,为了进一步探索 LplA 介导的抗体药物偶联物 (ADC) 合成,我们对使用 LplA 合成的 ADC 进行了初步生物学评估。使用抗 HER2 抗体曲妥珠单抗,我们使用 LplA 引入辛酸叠氮化物,随后使用药物 DBCO-VC-PAB-MMAE 使用点击化学获得 ADC。使用 HER2 阳性 SKBR-3 和 HER2 阴性 MCF7 细胞评估合成的抗 HER2-ADC 的生物活性。其毒性和选择性与 FDA 批准的 Kadcyla 相当。此外,一项涉及大鼠和人血浆的稳定性研究证明了 LplA 介导的 ADC 的稳定性。此外,接合后保留了对新生儿 Fc 受体 (FcRn) 的亲和力。这些初步体外评估表明,LplA 衍生的 ADC 具有相当大的药物潜力。我们的结果可以为进一步的体内评估和安全性评估奠定基础。我们建议将无标签 LplA 方法整合到 ADC 的生产中可以为生物制药制造提供一种新颖且有前景的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号