Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Intrinsically disordered Pseudomonas chaperone FapA slows down the fibrillation of major biofilm-forming functional amyloid FapC
The FEBS Journal ( IF 5.4 ) Pub Date : 2024-02-13 , DOI: 10.1111/febs.17084 Chang‐Hyeock Byeon 1 , Kasper Holst Hansen 1, 2 , Jasper Jeffrey 1 , Hakan Saricayir 1 , Maria Andreasen 2 , Ümit Akbey 1
The FEBS Journal ( IF 5.4 ) Pub Date : 2024-02-13 , DOI: 10.1111/febs.17084 Chang‐Hyeock Byeon 1 , Kasper Holst Hansen 1, 2 , Jasper Jeffrey 1 , Hakan Saricayir 1 , Maria Andreasen 2 , Ümit Akbey 1
Affiliation
|
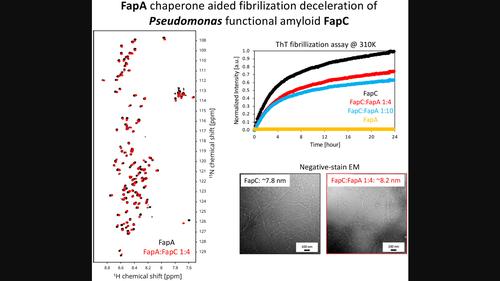
Functional bacterial amyloids play a crucial role in the formation of biofilms, which mediate chronic infections and contribute to antimicrobial resistance. This study focuses on the FapC amyloid fibrillar protein from Pseudomonas, a major contributor to biofilm formation. We investigate the initial steps of FapC amyloid formation and the impact of the chaperone-like protein FapA on this process. Using solution nuclear magnetic resonance (NMR), we recently showed that both FapC and FapA are intrinsically disordered proteins (IDPs). Here, the secondary structure propensities (SSPs) are compared to alphafold (DeepMind, protein structure prediction tool/algorithm: https://alphafold.ebi.ac.uk/) models. We further demonstrate that the FapA chaperone interacts with FapC and significantly slows down the formation of FapC fibrils. Our NMR titrations reveal ~ 18% of the resonances show FapA-induced chemical shift perturbations (CSPs), which has not been previously observed, the largest being for A82, N201, C237, C240, A241, and G245. These sites may suggest a specific interaction site and/or hotspots of fibrillation inhibition/control interface at the repeat-1 (R1)/loop-2 (L2) and L2/R3 transition areas and at the C-terminus of FapC. Remarkably, ~ 90% of FapA NMR signals exhibit substantial CSPs upon titration with FapC, the largest being for S63, A69, A80, and I92. A temperature-dependent effect of FapA was observed on FapC by thioflavin T (ThT) and NMR experiments. This study provides a detailed understanding of the interaction between the FapA and FapC, shedding light on the regulation and slowing down of amyloid formation, and has important implications for the development of therapeutic strategies targeting biofilms and associated infections.
中文翻译:

内在紊乱的假单胞菌伴侣 FapA 减缓主要生物膜形成功能性淀粉样蛋白 FapC 的纤维颤动
功能性细菌淀粉样蛋白在生物膜的形成中发挥着至关重要的作用,生物膜介导慢性感染并导致抗菌素耐药性。本研究重点关注假单胞菌中的 FapC 淀粉样纤维蛋白,它是生物膜形成的主要贡献者。我们研究了 FapC 淀粉样蛋白形成的初始步骤以及类伴侣蛋白 FapA 对此过程的影响。使用溶液核磁共振 (NMR),我们最近表明 FapC 和 FapA 都是本质上无序的蛋白质 (IDP)。此处,将二级结构倾向 (SSP) 与alphafold(DeepMind,蛋白质结构预测工具/算法:https://alphafold.ebi.ac.uk/)模型进行比较。我们进一步证明 FapA 伴侣与 FapC 相互作用并显着减缓 FapC 原纤维的形成。我们的 NMR 滴定显示约 18% 的共振显示 FapA 诱导的化学位移扰动 (CSP),这是以前未观察到的,最大的是 A82、N201、C237、C240、A241 和 G245。这些位点可能表明重复 1 (R1)/环 2 (L2) 和 L2/R3 过渡区域以及 FapC C 末端的颤动抑制/控制界面的特定相互作用位点和/或热点。值得注意的是,约 90% 的 FapA NMR 信号在用 FapC 滴定时表现出大量的 CSP,其中最大的是 S63、A69、A80 和 I92。通过硫代黄素 T (ThT) 和 NMR 实验观察到 FapA 对 FapC 的温度依赖性影响。这项研究提供了对 FapA 和 FapC 之间相互作用的详细了解,揭示了淀粉样蛋白形成的调节和减慢,并对针对生物膜和相关感染的治疗策略的开发具有重要意义。
更新日期:2024-02-13
中文翻译:

内在紊乱的假单胞菌伴侣 FapA 减缓主要生物膜形成功能性淀粉样蛋白 FapC 的纤维颤动
功能性细菌淀粉样蛋白在生物膜的形成中发挥着至关重要的作用,生物膜介导慢性感染并导致抗菌素耐药性。本研究重点关注假单胞菌中的 FapC 淀粉样纤维蛋白,它是生物膜形成的主要贡献者。我们研究了 FapC 淀粉样蛋白形成的初始步骤以及类伴侣蛋白 FapA 对此过程的影响。使用溶液核磁共振 (NMR),我们最近表明 FapC 和 FapA 都是本质上无序的蛋白质 (IDP)。此处,将二级结构倾向 (SSP) 与alphafold(DeepMind,蛋白质结构预测工具/算法:https://alphafold.ebi.ac.uk/)模型进行比较。我们进一步证明 FapA 伴侣与 FapC 相互作用并显着减缓 FapC 原纤维的形成。我们的 NMR 滴定显示约 18% 的共振显示 FapA 诱导的化学位移扰动 (CSP),这是以前未观察到的,最大的是 A82、N201、C237、C240、A241 和 G245。这些位点可能表明重复 1 (R1)/环 2 (L2) 和 L2/R3 过渡区域以及 FapC C 末端的颤动抑制/控制界面的特定相互作用位点和/或热点。值得注意的是,约 90% 的 FapA NMR 信号在用 FapC 滴定时表现出大量的 CSP,其中最大的是 S63、A69、A80 和 I92。通过硫代黄素 T (ThT) 和 NMR 实验观察到 FapA 对 FapC 的温度依赖性影响。这项研究提供了对 FapA 和 FapC 之间相互作用的详细了解,揭示了淀粉样蛋白形成的调节和减慢,并对针对生物膜和相关感染的治疗策略的开发具有重要意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号