Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Myosin heavy chain-perinatal regulates skeletal muscle differentiation, oxidative phenotype and regeneration
The FEBS Journal ( IF 5.4 ) Pub Date : 2024-02-15 , DOI: 10.1111/febs.17085 Akashi Sharma 1, 2 , Aatifa Zehra 1 , Sam J. Mathew 1, 2
The FEBS Journal ( IF 5.4 ) Pub Date : 2024-02-15 , DOI: 10.1111/febs.17085 Akashi Sharma 1, 2 , Aatifa Zehra 1 , Sam J. Mathew 1, 2
Affiliation
|
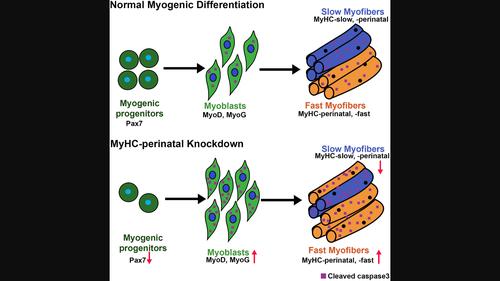
Myosin heavy chain-perinatal (MyHC-perinatal) is one of two development-specific myosin heavy chains expressed exclusively during skeletal muscle development and regeneration. The specific functions of MyHC-perinatal are unclear, although mutations are known to lead to contracture syndromes such as Trismus-pseudocamptodactyly syndrome. Here, we characterize the functions of MyHC-perinatal during skeletal muscle differentiation and regeneration. Loss of MyHC-perinatal function leads to enhanced differentiation characterized by increased expression of myogenic regulatory factors and differentiation index as well as reduced reserve cell numbers in vitro. Proteomic analysis revealed that loss of MyHC-perinatal function results in a switch from oxidative to glycolytic metabolism in myofibers, suggesting a shift from slow type I to fast type IIb fiber type, also supported by reduced mitochondrial numbers. Paracrine signals mediate the effect of loss of MyHC-perinatal function on myogenic differentiation, possibly mediated by non-apoptotic caspase-3 signaling along with enhanced levels of the pro-survival apoptosis regulator Bcl2 and nuclear factor kappa-B (NF-κB). Knockdown of MyHC-perinatal during muscle regeneration in vivo results in increased expression of the differentiation marker myogenin (MyoG) and impaired differentiation, evidenced by smaller myofibers, elevated fibrosis and reduction in the number of satellite cells. Thus, we find that MyHC-perinatal is a crucial regulator of myogenic differentiation, myofiber oxidative phenotype and regeneration.
中文翻译:

肌球蛋白重链-围产期调节骨骼肌分化、氧化表型和再生
围产期肌球蛋白重链 (MyHC-围产期) 是在骨骼肌发育和再生过程中专门表达的两条发育特异性肌球蛋白重链之一。MyHC-围产期的具体功能尚不清楚,尽管已知突变会导致挛缩综合征,例如牙关紧闭-假屈指综合征。在这里,我们描述了 MyHC-围产期在骨骼肌分化和再生过程中的功能。MyHC-围产期功能的丧失导致分化增强,其特征是生肌调节因子和分化指数表达增加以及体外储备细胞数量减少。蛋白质组学分析显示,MyHC 围产期功能的丧失导致肌纤维从氧化代谢转变为糖酵解代谢,表明从慢速 I 型纤维类型转变为快速 IIb 型纤维类型,这也得到线粒体数量减少的支持。旁分泌信号介导 MyHC 围产期功能丧失对肌原性分化的影响,可能是由非凋亡 caspase-3 信号传导以及促存活凋亡调节因子 Bcl2 和核因子 kappa-B (NF-κB) 水平升高介导的。体内肌肉再生过程中 MyHC-围产期的敲低会导致分化标记物肌细胞生成素 (MyoG) 表达增加和分化受损,表现为肌纤维变小、纤维化加剧和卫星细胞数量减少。因此,我们发现 MyHC-围产期是肌原分化、肌纤维氧化表型和再生的关键调节因子。
更新日期:2024-02-20
中文翻译:

肌球蛋白重链-围产期调节骨骼肌分化、氧化表型和再生
围产期肌球蛋白重链 (MyHC-围产期) 是在骨骼肌发育和再生过程中专门表达的两条发育特异性肌球蛋白重链之一。MyHC-围产期的具体功能尚不清楚,尽管已知突变会导致挛缩综合征,例如牙关紧闭-假屈指综合征。在这里,我们描述了 MyHC-围产期在骨骼肌分化和再生过程中的功能。MyHC-围产期功能的丧失导致分化增强,其特征是生肌调节因子和分化指数表达增加以及体外储备细胞数量减少。蛋白质组学分析显示,MyHC 围产期功能的丧失导致肌纤维从氧化代谢转变为糖酵解代谢,表明从慢速 I 型纤维类型转变为快速 IIb 型纤维类型,这也得到线粒体数量减少的支持。旁分泌信号介导 MyHC 围产期功能丧失对肌原性分化的影响,可能是由非凋亡 caspase-3 信号传导以及促存活凋亡调节因子 Bcl2 和核因子 kappa-B (NF-κB) 水平升高介导的。体内肌肉再生过程中 MyHC-围产期的敲低会导致分化标记物肌细胞生成素 (MyoG) 表达增加和分化受损,表现为肌纤维变小、纤维化加剧和卫星细胞数量减少。因此,我们发现 MyHC-围产期是肌原分化、肌纤维氧化表型和再生的关键调节因子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号