当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrogen/Deuterium Exchange Mass Spectrometry Provides Insights into the Role of Drosophila Testis-Specific Myosin VI Light Chain AndroCaM
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-15 , DOI: 10.1021/acs.biochem.3c00618 Jing Li 1 , Prashant N. Jethva 1 , Henry W. Rohrs 1 , Saketh Chemuru 1 , Kathryn Miller 2 , Michael L. Gross 1 , Kathleen M. Beckingham 3
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-15 , DOI: 10.1021/acs.biochem.3c00618 Jing Li 1 , Prashant N. Jethva 1 , Henry W. Rohrs 1 , Saketh Chemuru 1 , Kathryn Miller 2 , Michael L. Gross 1 , Kathleen M. Beckingham 3
Affiliation
|
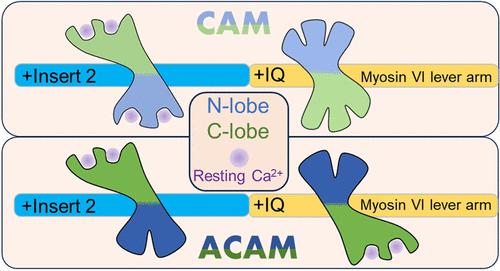
In Drosophila testis, myosin VI plays a special role, distinct from its motor function, by anchoring components to the unusual actin-based structures (cones) that are required for spermatid individualization. For this, the two calmodulin (CaM) light-chain molecules of myosin VI are replaced by androcam (ACaM), a related protein with 67% identity to CaM. Although ACaM has a similar bi-lobed structure to CaM, with two EF hand-type Ca2+ binding sites per lobe, only one functional Ca2+ binding site operates in the amino-terminus. To understand this light chain substitution, we used hydrogen–deuterium exchange mass spectrometry (HDX-MS) to examine dynamic changes in ACaM and CaM upon Ca2+ binding and interaction with the two CaM binding motifs of myosin VI (insert2 and IQ motif). HDX-MS reveals that binding of Ca2+ to ACaM destabilizes its N-lobe but stabilizes the entire C-lobe, whereas for CaM, Ca2+ binding induces a pattern of alternating stabilization/destabilization throughout. The conformation of this stable holo-C-lobe of ACaM seems to be a “prefigured” version of the conformation adopted by the holo-C-lobe of CaM for binding to insert2 and the IQ motif of myosin VI. Strikingly, the interaction of holo-ACaM with either peptide converts the holo-N-lobe to its Ca2+-free, more stable, form. Thus, ACaM in vivo should bind the myosin VI light chain sites in an apo-N-lobe/holo-C-lobe state that cannot fulfill the Ca2+-related functions of holo-CaM required for myosin VI motor assembly and activity. These findings indicate that inhibition of myosin VI motor activity is a precondition for transition to an anchoring function.
中文翻译:

氢/氘交换质谱可深入了解果蝇睾丸特异性肌球蛋白 VI 轻链 AndroCaM 的作用
在果蝇睾丸中,肌球蛋白 VI 发挥着不同于其运动功能的特殊作用,它将成分锚定到精细胞个体化所需的不寻常的基于肌动蛋白的结构(锥体)上。为此,肌球蛋白 VI 的两个钙调蛋白 (CaM) 轻链分子被 androcam (ACaM) 取代,这是一种与 CaM 具有 67% 同一性的相关蛋白。尽管 ACaM 具有与 CaM 类似的双叶结构,每个叶具有两个 EF 手型 Ca 2+结合位点,但只有一个功能性 Ca 2+结合位点在氨基末端起作用。为了了解这种轻链取代,我们使用氢-氘交换质谱 (HDX-MS) 来检查 ACaM 和 CaM 在 Ca 2+结合以及与肌球蛋白 VI 的两个 CaM 结合基序(insert2 和 IQ 基序)相互作用时的动态变化。HDX-MS 揭示,Ca 2+与 ACaM 的结合会使其 N 叶不稳定,但会稳定整个 C 叶,而对于 CaM,Ca 2+结合会诱导整个过程中交替稳定/不稳定的模式。ACaM 的这种稳定的全 C 叶构象似乎是 CaM 的全 C 叶所采用的构象的“预先设定”版本,用于结合插入物 2 和肌球蛋白 VI 的 IQ 基序。引人注目的是,holo-ACaM 与任一肽的相互作用将 Holo-N-lobe 转化为其不含 Ca 2+的更稳定的形式。因此,体内ACaM应以apo-N-lobe/holo-C-lobe状态结合肌球蛋白VI轻链位点,该状态不能实现肌球蛋白VI运动组装和活动所需的holo-CaM的Ca 2+相关功能。这些发现表明,抑制肌球蛋白 VI 运动活动是向锚定功能转变的先决条件。
更新日期:2024-02-15
中文翻译:

氢/氘交换质谱可深入了解果蝇睾丸特异性肌球蛋白 VI 轻链 AndroCaM 的作用
在果蝇睾丸中,肌球蛋白 VI 发挥着不同于其运动功能的特殊作用,它将成分锚定到精细胞个体化所需的不寻常的基于肌动蛋白的结构(锥体)上。为此,肌球蛋白 VI 的两个钙调蛋白 (CaM) 轻链分子被 androcam (ACaM) 取代,这是一种与 CaM 具有 67% 同一性的相关蛋白。尽管 ACaM 具有与 CaM 类似的双叶结构,每个叶具有两个 EF 手型 Ca 2+结合位点,但只有一个功能性 Ca 2+结合位点在氨基末端起作用。为了了解这种轻链取代,我们使用氢-氘交换质谱 (HDX-MS) 来检查 ACaM 和 CaM 在 Ca 2+结合以及与肌球蛋白 VI 的两个 CaM 结合基序(insert2 和 IQ 基序)相互作用时的动态变化。HDX-MS 揭示,Ca 2+与 ACaM 的结合会使其 N 叶不稳定,但会稳定整个 C 叶,而对于 CaM,Ca 2+结合会诱导整个过程中交替稳定/不稳定的模式。ACaM 的这种稳定的全 C 叶构象似乎是 CaM 的全 C 叶所采用的构象的“预先设定”版本,用于结合插入物 2 和肌球蛋白 VI 的 IQ 基序。引人注目的是,holo-ACaM 与任一肽的相互作用将 Holo-N-lobe 转化为其不含 Ca 2+的更稳定的形式。因此,体内ACaM应以apo-N-lobe/holo-C-lobe状态结合肌球蛋白VI轻链位点,该状态不能实现肌球蛋白VI运动组装和活动所需的holo-CaM的Ca 2+相关功能。这些发现表明,抑制肌球蛋白 VI 运动活动是向锚定功能转变的先决条件。



























 京公网安备 11010802027423号
京公网安备 11010802027423号