当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Using Vibrio natriegens for High-Yield Production of Challenging Expression Targets and for Protein Perdeuteration
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-15 , DOI: 10.1021/acs.biochem.3c00612 Natalia Mojica 1 , Flore Kersten 1, 2 , Mateu Montserrat-Canals 1, 2 , G. Robb Huhn III 3 , Abelone M. Tislevoll 1 , Gabriele Cordara 1 , Ken Teter 3 , Ute Krengel 1
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-15 , DOI: 10.1021/acs.biochem.3c00612 Natalia Mojica 1 , Flore Kersten 1, 2 , Mateu Montserrat-Canals 1, 2 , G. Robb Huhn III 3 , Abelone M. Tislevoll 1 , Gabriele Cordara 1 , Ken Teter 3 , Ute Krengel 1
Affiliation
|
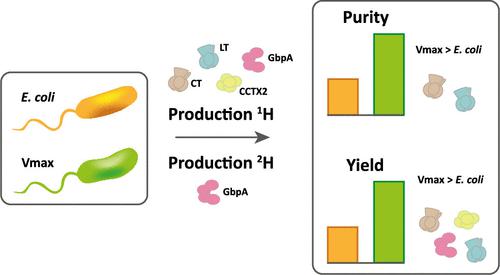
Production of soluble proteins is essential for structure/function studies; however, this usually requires milligram amounts of protein, which can be difficult to obtain with traditional expression systems. Recently, the Gram-negative bacterium Vibrio natriegens emerged as a novel and alternative host platform for production of proteins in high yields. Here, we used a commercial strain derived from V. natriegens (Vmax X2) to produce soluble bacterial and fungal proteins in milligram scale, which we struggled to achieve in Escherichia coli. These proteins include the cholera toxin (CT) and N-acetyl glucosamine-binding protein A (GbpA) from Vibrio cholerae, the heat-labile enterotoxin (LT) from E. coli and the fungal nematotoxin CCTX2 from Coprinopsis cinerea. CT, GbpA, and LT are secreted by the Type II secretion system in their natural hosts. When these three proteins were produced in Vmax, they were also secreted and could be recovered from the growth media. This simplified the downstream purification procedure and resulted in considerably higher protein yields compared to production in E. coli (6- to 26-fold increase). We also tested Vmax for protein perdeuteration using deuterated minimal media with deuterium oxide as solvent and achieved a 3-fold increase in yield compared to the equivalent protocol in E. coli. This is good news, since isotopic labeling is expensive and often ineffective but represents a necessary prerequisite for some structural biology techniques. Thus, Vmax represents a promising host for production of challenging expression targets and for protein perdeuteration in amounts suitable for structural biology studies.
中文翻译:

使用纳特里根弧菌高产生产具有挑战性的表达靶标并进行蛋白质全氘化
可溶性蛋白质的生产对于结构/功能研究至关重要;然而,这通常需要毫克量的蛋白质,而用传统的表达系统很难获得。最近,革兰氏阴性细菌Natriegens成为一种新型的替代宿主平台,用于高产蛋白质生产。在这里,我们使用源自V. natriegens (Vmax X2) 的商业菌株来生产毫克级的可溶性细菌和真菌蛋白,这是我们在大肠杆菌中努力实现的。这些蛋白质包括来自霍乱弧菌的霍乱毒素 (CT) 和N-乙酰氨基葡萄糖结合蛋白 A (GbpA) 、来自大肠杆菌的不耐热肠毒素 (LT)和来自灰鬼伞的真菌线虫毒素 CCTX2 。CT、GbpA 和 LT 由其天然宿主的 II 型分泌系统分泌。当这三种蛋白质在 Vmax 中产生时,它们也会被分泌并可以从生长培养基中回收。这简化了下游纯化程序,并且与大肠杆菌中的产量相比显着提高了蛋白质产量(增加了 6 至 26 倍)。我们还使用以氧化氘为溶剂的氘化基本培养基测试了蛋白质全氘化的 Vmax,与大肠杆菌中的等效方案相比,产量提高了 3 倍。这是个好消息,因为同位素标记价格昂贵且通常无效,但它是某些结构生物学技术的必要先决条件。因此,Vmax 代表了一种有前途的宿主,可用于生产具有挑战性的表达靶标以及以适合结构生物学研究的量进行蛋白质全氘化。
更新日期:2024-02-15
中文翻译:

使用纳特里根弧菌高产生产具有挑战性的表达靶标并进行蛋白质全氘化
可溶性蛋白质的生产对于结构/功能研究至关重要;然而,这通常需要毫克量的蛋白质,而用传统的表达系统很难获得。最近,革兰氏阴性细菌Natriegens成为一种新型的替代宿主平台,用于高产蛋白质生产。在这里,我们使用源自V. natriegens (Vmax X2) 的商业菌株来生产毫克级的可溶性细菌和真菌蛋白,这是我们在大肠杆菌中努力实现的。这些蛋白质包括来自霍乱弧菌的霍乱毒素 (CT) 和N-乙酰氨基葡萄糖结合蛋白 A (GbpA) 、来自大肠杆菌的不耐热肠毒素 (LT)和来自灰鬼伞的真菌线虫毒素 CCTX2 。CT、GbpA 和 LT 由其天然宿主的 II 型分泌系统分泌。当这三种蛋白质在 Vmax 中产生时,它们也会被分泌并可以从生长培养基中回收。这简化了下游纯化程序,并且与大肠杆菌中的产量相比显着提高了蛋白质产量(增加了 6 至 26 倍)。我们还使用以氧化氘为溶剂的氘化基本培养基测试了蛋白质全氘化的 Vmax,与大肠杆菌中的等效方案相比,产量提高了 3 倍。这是个好消息,因为同位素标记价格昂贵且通常无效,但它是某些结构生物学技术的必要先决条件。因此,Vmax 代表了一种有前途的宿主,可用于生产具有挑战性的表达靶标以及以适合结构生物学研究的量进行蛋白质全氘化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号