当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Biochemical Impact of Extracting an Embedded Adenylate Kinase Domain Using Circular Permutation
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-15 , DOI: 10.1021/acs.biochem.3c00605 Tom Coleman 1 , John Shin 1 , Jonathan J. Silberg 1, 2, 3 , Yousif Shamoo 1 , Joshua T. Atkinson 1, 4, 5, 6
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-15 , DOI: 10.1021/acs.biochem.3c00605 Tom Coleman 1 , John Shin 1 , Jonathan J. Silberg 1, 2, 3 , Yousif Shamoo 1 , Joshua T. Atkinson 1, 4, 5, 6
Affiliation
|
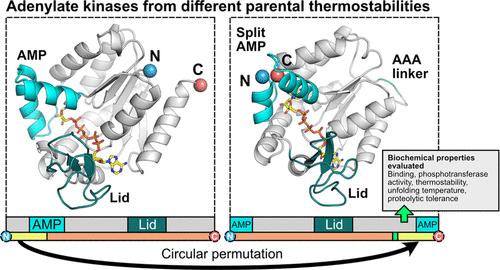
Adenylate kinases (AKs) have evolved AMP-binding and lid domains that are encoded as continuous polypeptides embedded at different locations within the discontinuous polypeptide encoding the core domain. A prior study showed that AK homologues of different stabilities consistently retain cellular activity following circular permutation that splits a region with high energetic frustration within the AMP-binding domain into discontinuous fragments. Herein, we show that mesophilic and thermophilic AKs having this topological restructuring retain activity and substrate-binding characteristics of the parental AK. While permutation decreased the activity of both AK homologues at physiological temperatures, the catalytic activity of the thermophilic AK increased upon permutation when assayed >30 °C below the melting temperature of the native AK. The thermostabilities of the permuted AKs were uniformly lower than those of native AKs, and they exhibited multiphasic unfolding transitions, unlike the native AKs, which presented cooperative thermal unfolding. In addition, proteolytic digestion revealed that permutation destabilized each AK in differing manners, and mass spectrometry suggested that the new termini within the AMP-binding domain were responsible for the increased proteolysis sensitivity. These findings illustrate how changes in contact order can be used to tune enzyme activity and alter folding dynamics in multidomain enzymes.
中文翻译:

使用循环排列提取嵌入式腺苷酸激酶结构域的生化影响
腺苷酸激酶 (AK) 已进化出 AMP 结合结构域和盖结构域,它们被编码为嵌入在编码核心结构域的不连续多肽内不同位置的连续多肽。先前的一项研究表明,不同稳定性的 AK 同源物在循环排列后始终保持细胞活性,循环排列将 AMP 结合域内具有高能量挫败的区域分割成不连续的片段。在此,我们证明具有这种拓扑重组的嗜温和嗜热 AK 保留了亲本 AK 的活性和底物结合特征。虽然排列降低了两种 AK 同系物在生理温度下的活性,但当在比天然 AK 熔点温度低 > 30 °C 的温度进行测定时,嗜热 AK 的催化活性在排列后增加。排列后的 AK 的热稳定性均低于天然 AK,并且与呈现协同热解折叠的天然 AK 不同,它们表现出多相解折叠转变。此外,蛋白水解消化显示排列以不同方式破坏了每个 AK 的稳定性,质谱分析表明 AMP 结合结构域内的新末端导致蛋白水解敏感性增加。这些发现说明了如何利用接触顺序的变化来调节酶活性并改变多域酶的折叠动力学。
更新日期:2024-02-15
中文翻译:

使用循环排列提取嵌入式腺苷酸激酶结构域的生化影响
腺苷酸激酶 (AK) 已进化出 AMP 结合结构域和盖结构域,它们被编码为嵌入在编码核心结构域的不连续多肽内不同位置的连续多肽。先前的一项研究表明,不同稳定性的 AK 同源物在循环排列后始终保持细胞活性,循环排列将 AMP 结合域内具有高能量挫败的区域分割成不连续的片段。在此,我们证明具有这种拓扑重组的嗜温和嗜热 AK 保留了亲本 AK 的活性和底物结合特征。虽然排列降低了两种 AK 同系物在生理温度下的活性,但当在比天然 AK 熔点温度低 > 30 °C 的温度进行测定时,嗜热 AK 的催化活性在排列后增加。排列后的 AK 的热稳定性均低于天然 AK,并且与呈现协同热解折叠的天然 AK 不同,它们表现出多相解折叠转变。此外,蛋白水解消化显示排列以不同方式破坏了每个 AK 的稳定性,质谱分析表明 AMP 结合结构域内的新末端导致蛋白水解敏感性增加。这些发现说明了如何利用接触顺序的变化来调节酶活性并改变多域酶的折叠动力学。



























 京公网安备 11010802027423号
京公网安备 11010802027423号