Synthesis ( IF 2.6 ) Pub Date : 2024-02-15 , DOI: 10.1055/s-0042-1751554 Virginie Blot 1 , Hédi M’rabet 2 , Momtez Jmai 1, 2 , Monique Mathé-Allainmat 1 , Mohamed Lotfi Efrit 2 , Didier Dubreuil 1 , Jacques Lebreton 1
|
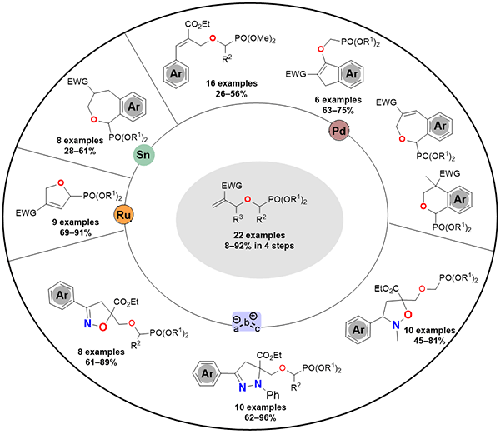
This paper describes the versatility of substituted [(allyloxy)methyl]phosphonates to open the way to the synthesis of original phosphonated molecules with heterocyclic architectures. In 1,3-dipolar cycloaddition reactions with nitrile oxides, nitrile imines, and nitrones, these [(allyloxy)methyl]phosphonates react as dipolarophiles to give, regioselectively, the corresponding isoxazolines, pyrazolines, and isoxazolidines. Transition-metal-catalyzed reactions, including inter- or intramolecular Heck coupling, provided access to cinnamyl- and indenyl-linked moieties and phosphonated benzo-fused oxacycles, respectively. Additionally, ring-closing metathesis reactions enabled the synthesis of 2,5-dihydrofurans with the phosphonate group at the anomeric position. In this work, 51 novel phosphorylated heterocyclic compounds, which may find significance in the pharmaceutical and agrochemical fields, were prepared.
中文翻译:

探索取代的[(烯丙氧基)甲基]膦酸酯在环加成和偶联反应中的反应性
本文描述了取代的[(烯丙氧基)甲基]膦酸酯的多功能性,为合成具有杂环结构的原始膦酸化分子开辟了道路。在与腈氧化物、腈亚胺和硝酮的 1,3-偶极环加成反应中,这些[(烯丙氧基)甲基]膦酸酯作为亲偶极试剂反应,区域选择性地生成相应的异恶唑啉、吡唑啉和异恶唑烷。过渡金属催化反应,包括分子间或分子内 Heck 偶联,分别提供了肉桂基和茚基连接部分以及膦酸化苯并稠合氧杂环的途径。此外,闭环复分解反应使得能够合成在异头位置具有膦酸酯基团的2,5-二氢呋喃。在这项工作中,制备了51种新型磷酸化杂环化合物,这些化合物可能在医药和农化领域具有重要意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号