当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterization of a Myeloid Differentiation Factor 2-Derived Peptide that Facilitates THP-1 Macrophage-Mediated Phagocytosis of Gram-Negative Bacteria
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2024-02-16 , DOI: 10.1021/acsinfecdis.3c00274 Anshika Tandon 1 , Munesh Kumar Harioudh 1 , Neeraj Kumar Verma 1 , Jyotshana Saroj 1, 2 , Arvind Gupta 1, 2 , Garima Pant 3 , Jitendra Kumar Tripathi 1 , Amit Kumar 1 , Tripti Kumari 1 , Amit Kumar Tripathi 1 , Kalyan Mitra 3 , Jimut Kanti Ghosh 1, 2
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2024-02-16 , DOI: 10.1021/acsinfecdis.3c00274 Anshika Tandon 1 , Munesh Kumar Harioudh 1 , Neeraj Kumar Verma 1 , Jyotshana Saroj 1, 2 , Arvind Gupta 1, 2 , Garima Pant 3 , Jitendra Kumar Tripathi 1 , Amit Kumar 1 , Tripti Kumari 1 , Amit Kumar Tripathi 1 , Kalyan Mitra 3 , Jimut Kanti Ghosh 1, 2
Affiliation
|
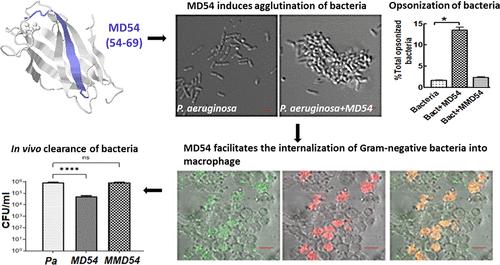
Myeloid differentiation factor 2 (MD2), the TLR4 coreceptor, has been shown to possess opsonic activity and has been implicated in phagocytosis and intracellular killing of Gram-negative bacteria. However, any MD2 protein segment involved in phagocytosis of Gram-negative bacteria is not yet known. A short synthetic MD2 segment, MD54 (amino acid regions 54 to 69), was shown to interact with a Gram-negative bacterial outer membrane component, LPS, earlier. Furthermore, the MD54 peptide induced aggregation of LPS and facilitated its internalization in THP-1 cells. Currently, it has been investigated if MD2-derived MD54 possesses any opsonic property and role in phagocytosis of Gram-negative bacteria. Remarkably, we observed that MD54 facilitated agglutination of Gram-negative bacteria, Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC BAA-427), but not of Gram-positive bacteria, Bacillus subtilis (ATCC 6633) and Staphylococcus aureus (ATCC 25923). The MD54-opsonized Gram-negative bacteria internalized within PMA-treated THP-1 cells and were killed over a longer incubation period. However, both internalization and intracellular killing of the MD54-opsonized Gram-negative bacteria within THP-1 phagocytes were appreciably inhibited in the presence of a phagocytosis inhibitor, cytochalasin D. Furthermore, MD54 facilitated the clearance of Gram-negative bacteria E. coli (ATCC 25922) and P. aeruginosa (ATCC BAA-427) from the infected BALB/c mice whereas an MD54 analog, MMD54, was inactive. Overall, for the first time, the results revealed that a short MD2-derived peptide can specifically agglutinate Gram-negative bacteria, act as an opsonin for these bacteria, and facilitate their phagocytosis by THP-1 phagocytes. The results suggest that the MD54 segment could have a crucial role in MD2-mediated host–pathogen interaction involving the Gram-negative bacteria.
中文翻译:

促进 THP-1 巨噬细胞介导的革兰氏阴性菌吞噬作用的骨髓分化因子 2 衍生肽的表征
骨髓分化因子 2 (MD2) 是 TLR4 的辅助受体,已被证明具有调理活性,并参与革兰氏阴性菌的吞噬作用和细胞内杀伤作用。然而,任何参与革兰氏阴性菌吞噬作用的 MD2 蛋白片段尚不清楚。较早的研究表明,一个短的合成 MD2 片段 MD54(氨基酸区域 54 至 69)可与革兰氏阴性细菌外膜成分 LPS 相互作用。此外,MD54 肽诱导 LPS 聚集并促进其在 THP-1 细胞中的内化。目前,人们正在研究MD2衍生的MD54是否具有任何调理特性以及在革兰氏阴性菌的吞噬作用中的作用。值得注意的是,我们观察到 MD54 促进革兰氏阴性菌、大肠杆菌(ATCC 25922) 和铜绿假单胞菌(ATCC BAA-427) 的凝集,但不促进革兰氏阳性菌、枯草芽孢杆菌(ATCC 6633) 和金黄色葡萄球菌(ATCC 25923) 的凝集。 )。MD54 调理的革兰氏阴性细菌内化于 PMA 处理的 THP-1 细胞内,并在较长的潜伏期内被杀死。然而,在吞噬作用抑制剂细胞松弛素 D 的存在下,THP-1 吞噬细胞内 MD54 调理的革兰氏阴性菌的内化和细胞内杀伤均受到明显抑制。此外,MD54 促进了革兰氏阴性菌大肠杆菌的清除。 ATCC 25922) 和铜绿假单胞菌(ATCC BAA-427) 来自受感染的 BALB/c 小鼠,而 MD54 类似物 MMD54 则无活性。总体而言,结果首次揭示了短的 MD2 衍生肽可以特异性凝集革兰氏阴性细菌,充当这些细菌的调理素,并促进 THP-1 吞噬细胞的吞噬作用。结果表明,MD54 片段可能在涉及革兰氏阴性菌的 MD2 介导的宿主-病原体相互作用中发挥至关重要的作用。
更新日期:2024-02-16
中文翻译:

促进 THP-1 巨噬细胞介导的革兰氏阴性菌吞噬作用的骨髓分化因子 2 衍生肽的表征
骨髓分化因子 2 (MD2) 是 TLR4 的辅助受体,已被证明具有调理活性,并参与革兰氏阴性菌的吞噬作用和细胞内杀伤作用。然而,任何参与革兰氏阴性菌吞噬作用的 MD2 蛋白片段尚不清楚。较早的研究表明,一个短的合成 MD2 片段 MD54(氨基酸区域 54 至 69)可与革兰氏阴性细菌外膜成分 LPS 相互作用。此外,MD54 肽诱导 LPS 聚集并促进其在 THP-1 细胞中的内化。目前,人们正在研究MD2衍生的MD54是否具有任何调理特性以及在革兰氏阴性菌的吞噬作用中的作用。值得注意的是,我们观察到 MD54 促进革兰氏阴性菌、大肠杆菌(ATCC 25922) 和铜绿假单胞菌(ATCC BAA-427) 的凝集,但不促进革兰氏阳性菌、枯草芽孢杆菌(ATCC 6633) 和金黄色葡萄球菌(ATCC 25923) 的凝集。 )。MD54 调理的革兰氏阴性细菌内化于 PMA 处理的 THP-1 细胞内,并在较长的潜伏期内被杀死。然而,在吞噬作用抑制剂细胞松弛素 D 的存在下,THP-1 吞噬细胞内 MD54 调理的革兰氏阴性菌的内化和细胞内杀伤均受到明显抑制。此外,MD54 促进了革兰氏阴性菌大肠杆菌的清除。 ATCC 25922) 和铜绿假单胞菌(ATCC BAA-427) 来自受感染的 BALB/c 小鼠,而 MD54 类似物 MMD54 则无活性。总体而言,结果首次揭示了短的 MD2 衍生肽可以特异性凝集革兰氏阴性细菌,充当这些细菌的调理素,并促进 THP-1 吞噬细胞的吞噬作用。结果表明,MD54 片段可能在涉及革兰氏阴性菌的 MD2 介导的宿主-病原体相互作用中发挥至关重要的作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号