当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalyzing Sustainable Water Splitting with Single Atom Catalysts: Recent Advances
The Chemical Record ( IF 6.6 ) Pub Date : 2024-02-19 , DOI: 10.1002/tcr.202300330 Nasar Alam 1 , Tayyaba Noor 1 , Naseem Iqbal 2
The Chemical Record ( IF 6.6 ) Pub Date : 2024-02-19 , DOI: 10.1002/tcr.202300330 Nasar Alam 1 , Tayyaba Noor 1 , Naseem Iqbal 2
Affiliation
|
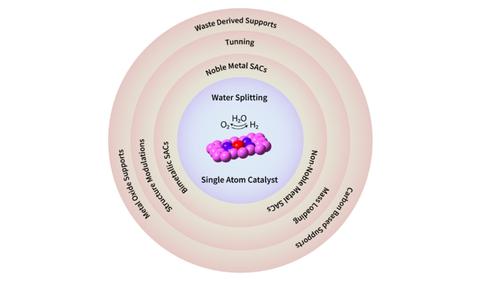
Electrochemical water splitting for sustainable hydrogen and oxygen production have shown enormous potentials. However, this method needs low-cost and highly active catalysts. Traditional nano catalysts, while effective, have limits since their active sites are mostly restricted to the surface and edges, leaving interior surfaces unexposed in redox reactions. Single atom catalysts (SACs), which take advantage of high atom utilization and quantum size effects, have recently become appealing electrocatalysts. Strong interaction between active sites and support in SACs have considerably improved the catalytic efficiency and long-term stability, outperforming their nano-counterparts. This review‘s first section examines the Hydrogen Evolution Reaction (HER) and the Oxygen Evolution Reaction (OER). In the next section, SACs are categorized as noble metal, non-noble metal, and bimetallic synergistic SACs. In addition, this review emphasizes developing methodologies for effective SAC design, such as mass loading optimization, electrical structure modulation, and the critical role of support materials. Finally, Carbon-based materials and metal oxides are being explored as possible supports for SACs. Importantly, for the first time, this review opens a discussion on waste-derived supports for single atom catalysts used in electrochemical reactions, providing a cost-effective dimension to this vibrant research field. The well-known design techniques discussed here may help in development of electrocatalysts for effective water splitting.
中文翻译:

用单原子催化剂催化可持续水分解:最新进展
用于可持续生产氢气和氧气的电化学水分解已显示出巨大的潜力。然而,该方法需要低成本和高活性的催化剂。传统的纳米催化剂虽然有效,但也有局限性,因为它们的活性位点大多局限于表面和边缘,导致内表面在氧化还原反应中不暴露。单原子催化剂(SAC)利用高原子利用率和量子尺寸效应,最近已成为颇具吸引力的电催化剂。SAC 中的活性位点和载体之间的强相互作用显着提高了催化效率和长期稳定性,优于纳米对应物。本综述的第一部分研究了析氢反应 (HER) 和析氧反应 (OER)。在下一节中,SAC 分为贵金属、非贵金属和双金属协同 SAC。此外,本次综述强调开发有效 SAC 设计的方法,例如质量负载优化、电气结构调制和支撑材料的关键作用。最后,人们正在探索碳基材料和金属氧化物作为 SAC 的可能载体。重要的是,这篇综述首次开启了关于电化学反应中使用的单原子催化剂的废物衍生载体的讨论,为这个充满活力的研究领域提供了具有成本效益的维度。这里讨论的众所周知的设计技术可能有助于开发有效水分解的电催化剂。
更新日期:2024-02-19
中文翻译:

用单原子催化剂催化可持续水分解:最新进展
用于可持续生产氢气和氧气的电化学水分解已显示出巨大的潜力。然而,该方法需要低成本和高活性的催化剂。传统的纳米催化剂虽然有效,但也有局限性,因为它们的活性位点大多局限于表面和边缘,导致内表面在氧化还原反应中不暴露。单原子催化剂(SAC)利用高原子利用率和量子尺寸效应,最近已成为颇具吸引力的电催化剂。SAC 中的活性位点和载体之间的强相互作用显着提高了催化效率和长期稳定性,优于纳米对应物。本综述的第一部分研究了析氢反应 (HER) 和析氧反应 (OER)。在下一节中,SAC 分为贵金属、非贵金属和双金属协同 SAC。此外,本次综述强调开发有效 SAC 设计的方法,例如质量负载优化、电气结构调制和支撑材料的关键作用。最后,人们正在探索碳基材料和金属氧化物作为 SAC 的可能载体。重要的是,这篇综述首次开启了关于电化学反应中使用的单原子催化剂的废物衍生载体的讨论,为这个充满活力的研究领域提供了具有成本效益的维度。这里讨论的众所周知的设计技术可能有助于开发有效水分解的电催化剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号