当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ring-opening polymerisation of alkyl-substituted ε-caprolactones: kinetic effects of substitution position
Polymer Chemistry ( IF 4.6 ) Pub Date : 2024-02-20 , DOI: 10.1039/d3py01380d Cinzia Clamor 1 , James Beament 2 , Peter M. Wright 2 , Beatrice N. Cattoz 2 , Rachel K. O'Reilly 1 , Andrew P. Dove 1
Polymer Chemistry ( IF 4.6 ) Pub Date : 2024-02-20 , DOI: 10.1039/d3py01380d Cinzia Clamor 1 , James Beament 2 , Peter M. Wright 2 , Beatrice N. Cattoz 2 , Rachel K. O'Reilly 1 , Andrew P. Dove 1
Affiliation
|
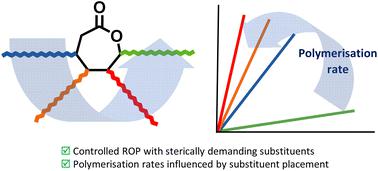
Ring-opening polymerisation (ROP) of lactones has been proven as a powerful technique to generate polyesters with high levels of control over molar mass and polymer dispersity. However, the introduction of functional groups on the monomer ring structure can dramatically influence the ability of a monomer to undergo ROP. Therefore, understanding the structure–reactivity relationship of functional monomers is essential to gain access to materials with chemical functionality via direct polymerisation. Herein, we report how structural modifications of alkyl-substituted ε-caprolactones affected their reactivity towards the ring-opening of the functional monomer. We observed that the reactivity was strongly influenced by the substituent position, wherein the δ-substituted monomer exhibited the fastest polymerisation kinetics. In contrast, a substituent placement in the ε-position significantly reduced polymerisation time compared to other substituent positions. Moreover, the thermal properties of the resultant functional ε-polycaprolactones were investigated and showed no significant change in the thermal transitions. This demonstrates that functional caprolactone monomers with sterically demanding functional groups can still undergo direct ring-opening polymerisation and that careful positioning of these functional groups enables control of the rate of polymerisation, a crucial parameter to be considered for the design of new prospective functional monomers and their industrial applications.
中文翻译:

烷基取代的ε-己内酯的开环聚合:取代位置的动力学效应
内酯的开环聚合 (ROP) 已被证明是一种强大的技术,可以生成对摩尔质量和聚合物分散性进行高度控制的聚酯。然而,在单体环结构上引入官能团可以显着影响单体进行 ROP 的能力。因此,了解功能单体的结构-反应性关系对于通过直接聚合获得具有化学功能的材料至关重要。在此,我们报道了烷基取代的ε-己内酯的结构修饰如何影响其对功能单体的开环反应性。我们观察到反应活性受到取代基位置的强烈影响,其中δ-取代单体表现出最快的聚合动力学。相反,与其他取代基位置相比,ε-位上的取代基显着缩短了聚合时间。此外,对所得功能性ε-聚己内酯的热性能进行了研究,结果表明热转变没有显着变化。这表明具有空间要求官能团的功能性己内酯单体仍然可以进行直接开环聚合,并且这些官能团的仔细定位能够控制聚合速率,这是设计新的预期功能性单体时需要考虑的关键参数他们的工业应用。
更新日期:2024-02-20
中文翻译:

烷基取代的ε-己内酯的开环聚合:取代位置的动力学效应
内酯的开环聚合 (ROP) 已被证明是一种强大的技术,可以生成对摩尔质量和聚合物分散性进行高度控制的聚酯。然而,在单体环结构上引入官能团可以显着影响单体进行 ROP 的能力。因此,了解功能单体的结构-反应性关系对于通过直接聚合获得具有化学功能的材料至关重要。在此,我们报道了烷基取代的ε-己内酯的结构修饰如何影响其对功能单体的开环反应性。我们观察到反应活性受到取代基位置的强烈影响,其中δ-取代单体表现出最快的聚合动力学。相反,与其他取代基位置相比,ε-位上的取代基显着缩短了聚合时间。此外,对所得功能性ε-聚己内酯的热性能进行了研究,结果表明热转变没有显着变化。这表明具有空间要求官能团的功能性己内酯单体仍然可以进行直接开环聚合,并且这些官能团的仔细定位能够控制聚合速率,这是设计新的预期功能性单体时需要考虑的关键参数他们的工业应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号