当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Insights into the Interaction between the C-Terminal-Deleted BH3-like Motif Peptide of Hepatitis B Virus X Protein and Bcl-xL
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-20 , DOI: 10.1021/acs.biochem.3c00709 Hideki Kusunoki 1 , Taiichi Sakamoto 2 , Naohiro Kobayashi 3 , Toshiyuki Kohno 4 , Kaori Wakamatsu 5 , Takashi Nagata 6, 7, 8, 9
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-20 , DOI: 10.1021/acs.biochem.3c00709 Hideki Kusunoki 1 , Taiichi Sakamoto 2 , Naohiro Kobayashi 3 , Toshiyuki Kohno 4 , Kaori Wakamatsu 5 , Takashi Nagata 6, 7, 8, 9
Affiliation
|
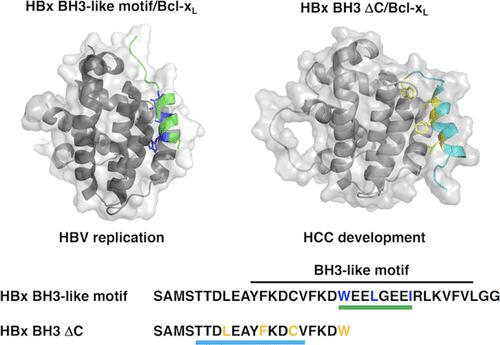
Hepatitis B virus X protein (HBx) plays a crucial role in the development of hepatocellular carcinoma (HCC) associated with hepatitis B virus (HBV) infection. The full-length HBx protein interacts with Bcl-xL and is involved in the HBV replication and cell death processes. The three hydrophobic residues Trp120, Leu123, and Ile127 of the HBx BH3-like motif are essential for the Bcl-xL-binding. On the other hand, various lengths of C-terminal-truncated HBx mutants are frequently detected in HCC tissues, and these mutants, rather than the full-length HBx, appear to be responsible for HCC development. Notably, the region spanning residues 1–120 of HBx [HBx(1 and 120)] has been strongly associated with an increased risk of HCC development. However, the mode of interaction between HBx(1–120) and Bcl-xL remains unclear. HBx(1–120) possesses only Trp120 among the three hydrophobic residues essential for the Bcl-xL-binding. To elucidate this interaction mode, we employed a C-terminal-deleted HBx BH3-like motif peptide composed of residues 101–120. Here, we present the NMR complex structure of Bcl-xL and HBx(101–120). Our results demonstrate that HBx(101–120) binds to Bcl-xL in a weaker manner. Considering the high expression of Bcl-xL in HCC cells, this weak interaction, in conjunction with the overexpression of Bcl-xL in HCC cells, may potentially contribute to HCC development through the interaction between C-terminal-truncated HBx and Bcl-xL.
中文翻译:

乙型肝炎病毒 X 蛋白 C 端缺失 BH3 样基序肽与 Bcl-xL 之间相互作用的结构见解
乙型肝炎病毒X蛋白(HBx)在与乙型肝炎病毒(HBV)感染相关的肝细胞癌(HCC)的发展中起着至关重要的作用。全长 HBx 蛋白与 Bcl-x L相互作用,参与 HBV 复制和细胞死亡过程。HBx BH3 样基序的三个疏水残基 Trp120、Leu123 和 Ile127 对于 Bcl-x L结合至关重要。另一方面,在 HCC 组织中经常检测到各种长度的 C 端截短的 HBx 突变体,这些突变体(而不是全长 HBx)似乎负责 HCC 的发展。值得注意的是,跨越 HBx 残基 1-120 的区域 [HBx(1 和 120)] 与 HCC 发展风险增加密切相关。然而,HBx(1-120) 和 Bcl-x L之间的相互作用模式仍不清楚。HBx(1–120) 在 Bcl-x L结合所必需的三个疏水残基中仅具有 Trp120。为了阐明这种相互作用模式,我们采用了由残基 101-120 组成的 C 端缺失的 HBx BH3 样基序肽。在这里,我们展示了 Bcl-x L和 HBx(101–120)的 NMR 复合结构。我们的结果表明 HBx(101–120)以较弱的方式与 Bcl-x L结合。考虑到 HCC 细胞中 Bcl-x L的高表达,这种弱相互作用与 HCC 细胞中 Bcl-x L的过度表达相结合,可能通过 C 端截短的 HBx 和 Bcl- 之间的相互作用促进 HCC 的发展。 x L。
更新日期:2024-02-20
中文翻译:

乙型肝炎病毒 X 蛋白 C 端缺失 BH3 样基序肽与 Bcl-xL 之间相互作用的结构见解
乙型肝炎病毒X蛋白(HBx)在与乙型肝炎病毒(HBV)感染相关的肝细胞癌(HCC)的发展中起着至关重要的作用。全长 HBx 蛋白与 Bcl-x L相互作用,参与 HBV 复制和细胞死亡过程。HBx BH3 样基序的三个疏水残基 Trp120、Leu123 和 Ile127 对于 Bcl-x L结合至关重要。另一方面,在 HCC 组织中经常检测到各种长度的 C 端截短的 HBx 突变体,这些突变体(而不是全长 HBx)似乎负责 HCC 的发展。值得注意的是,跨越 HBx 残基 1-120 的区域 [HBx(1 和 120)] 与 HCC 发展风险增加密切相关。然而,HBx(1-120) 和 Bcl-x L之间的相互作用模式仍不清楚。HBx(1–120) 在 Bcl-x L结合所必需的三个疏水残基中仅具有 Trp120。为了阐明这种相互作用模式,我们采用了由残基 101-120 组成的 C 端缺失的 HBx BH3 样基序肽。在这里,我们展示了 Bcl-x L和 HBx(101–120)的 NMR 复合结构。我们的结果表明 HBx(101–120)以较弱的方式与 Bcl-x L结合。考虑到 HCC 细胞中 Bcl-x L的高表达,这种弱相互作用与 HCC 细胞中 Bcl-x L的过度表达相结合,可能通过 C 端截短的 HBx 和 Bcl- 之间的相互作用促进 HCC 的发展。 x L。



























 京公网安备 11010802027423号
京公网安备 11010802027423号