Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Non-canonical amino acids uncover the significant impact of Tyr671 on Taq DNA polymerase catalytic activity
The FEBS Journal ( IF 5.4 ) Pub Date : 2024-02-16 , DOI: 10.1111/febs.17091 Wanyi Chen 1, 2 , Binbin Chen 1, 2 , Xinjia Li 1, 2 , Gang Xu 1 , Lirong Yang 1, 2 , Jianping Wu 1, 2 , Haoran Yu 1, 2
The FEBS Journal ( IF 5.4 ) Pub Date : 2024-02-16 , DOI: 10.1111/febs.17091 Wanyi Chen 1, 2 , Binbin Chen 1, 2 , Xinjia Li 1, 2 , Gang Xu 1 , Lirong Yang 1, 2 , Jianping Wu 1, 2 , Haoran Yu 1, 2
Affiliation
|
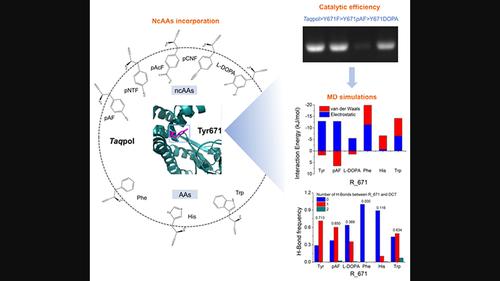
Responsible for synthesizing the complementary strand of the DNA template, DNA polymerase is a crucial enzyme in DNA replication, recombination and repair. A highly conserved tyrosine (Tyr), located at the C-terminus of the O-helix in family A DNA polymerases, plays a critical role in enzyme activity and fidelity. Here, we combined the technology of genetic code extension to incorporate non-canonical amino acids and molecular dynamics (MD) simulations to uncover the mechanisms by which Tyr671 impacts substrate binding and conformation transitions in a DNA polymerase from Thermus aquaticus. Five non-canonical amino acids, namely l-3,4-dihydroxyphenylalanine (l-DOPA), p-aminophenylalanine (pAF), p-acetylphenylalanine (pAcF), p-cyanophenylalanine (pCNF) and p-nitrophenylalanine (pNTF), were individually incorporated at position 671. Strikingly, Y671pAF and Y671DOPA were active, but with lower activity compared to Y671F and wild-type. Y671pAF showed a higher fidelity than the Y671F, despite both possessing lower fidelity than the wild-type. Metadynamics and long-timescale MD simulations were carried out to probe the role of mutations in affecting protein structure, including open conformation, open-to-closed conformation transition, closed conformation, and closed-to-open conformation transition. The MD simulations clearly revealed that the size of the 671 amino acid residue and interactions with substrate or nearby residues were critical for Tyr671 to determine enzyme activity and fidelity.
中文翻译:

非规范氨基酸揭示了 Tyr671 对 Taq DNA 聚合酶催化活性的显着影响
DNA聚合酶负责合成DNA模板的互补链,是DNA复制、重组和修复中的关键酶。高度保守的酪氨酸 (Tyr) 位于 A 家族 DNA 聚合酶 O 螺旋的 C 末端,在酶活性和保真度中发挥着关键作用。在这里,我们结合遗传密码延伸技术,将非规范氨基酸与分子动力学 (MD) 模拟结合起来,揭示了 Tyr671 影响来自栖热菌DNA 聚合酶的底物结合和构象转变的机制。五种非规范氨基酸,即l -3,4-二羟基苯丙氨酸 ( l -DOPA)、对氨基苯丙氨酸 ( p AF)、对乙酰基苯丙氨酸 ( p AcF)、对氰基苯丙氨酸 ( p CNF) 和对硝基苯丙氨酸 ( p NTF),分别掺入位置 671。引人注目的是,Y671 p AF 和 Y671DOPA 具有活性,但与 Y671F 和野生型相比活性较低。Y671 p AF 显示出比 Y671F 更高的保真度,尽管两者的保真度均低于野生型。通过元动力学和长时间尺度MD模拟来探讨突变在影响蛋白质结构中的作用,包括开放构象、开放到封闭构象转变、封闭构象和封闭到开放构象转变。MD 模拟清楚地表明,671 个氨基酸残基的大小以及与底物或附近残基的相互作用对于 Tyr671 确定酶活性和保真度至关重要。
更新日期:2024-02-20
中文翻译:

非规范氨基酸揭示了 Tyr671 对 Taq DNA 聚合酶催化活性的显着影响
DNA聚合酶负责合成DNA模板的互补链,是DNA复制、重组和修复中的关键酶。高度保守的酪氨酸 (Tyr) 位于 A 家族 DNA 聚合酶 O 螺旋的 C 末端,在酶活性和保真度中发挥着关键作用。在这里,我们结合遗传密码延伸技术,将非规范氨基酸与分子动力学 (MD) 模拟结合起来,揭示了 Tyr671 影响来自栖热菌DNA 聚合酶的底物结合和构象转变的机制。五种非规范氨基酸,即l -3,4-二羟基苯丙氨酸 ( l -DOPA)、对氨基苯丙氨酸 ( p AF)、对乙酰基苯丙氨酸 ( p AcF)、对氰基苯丙氨酸 ( p CNF) 和对硝基苯丙氨酸 ( p NTF),分别掺入位置 671。引人注目的是,Y671 p AF 和 Y671DOPA 具有活性,但与 Y671F 和野生型相比活性较低。Y671 p AF 显示出比 Y671F 更高的保真度,尽管两者的保真度均低于野生型。通过元动力学和长时间尺度MD模拟来探讨突变在影响蛋白质结构中的作用,包括开放构象、开放到封闭构象转变、封闭构象和封闭到开放构象转变。MD 模拟清楚地表明,671 个氨基酸残基的大小以及与底物或附近残基的相互作用对于 Tyr671 确定酶活性和保真度至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号