当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification and characterization of nuclear localization signals in the circumsporozoite protein of Plasmodium falciparum
FEBS Letters ( IF 3.5 ) Pub Date : 2024-02-18 , DOI: 10.1002/1873-3468.14829 Akshaykumar Nanaji Shrikondawar 1, 2 , Kiranmai Chennoju 1, 3 , Debasish Kumar Ghosh 4 , Akash Ranjan 1
FEBS Letters ( IF 3.5 ) Pub Date : 2024-02-18 , DOI: 10.1002/1873-3468.14829 Akshaykumar Nanaji Shrikondawar 1, 2 , Kiranmai Chennoju 1, 3 , Debasish Kumar Ghosh 4 , Akash Ranjan 1
Affiliation
|
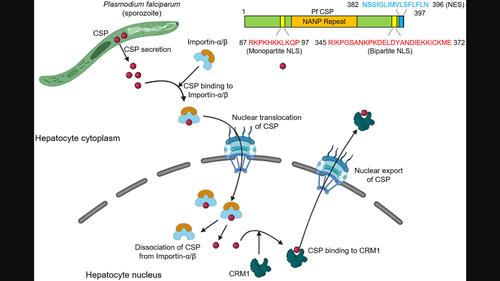
Secretory proteins of Plasmodium exhibit differential spatial and functional activity within the host cell nucleus. However, the nuclear localization signals (NLSs) for these proteins remain largely uncharacterized. In this study, we have identified and characterized two NLSs in the circumsporozoite protein of Plasmodium falciparum (Pf-CSP). Both NLSs in the Pf-CSP contain clusters of lysine and arginine residues essential for specific interactions with the conserved tryptophan and asparagine residues of importin-α, facilitating nuclear translocation of Pf-CSP. While the two NLSs of Pf-CSP function independently and are both crucial for nuclear localization, a single NLS of Pf-CSP leads to weak nuclear localization. These findings shed light on the mechanism of nuclear penetrability of secretory proteins of Plasmodium proteins.
中文翻译:

恶性疟原虫环子孢子蛋白核定位信号的鉴定和表征
疟原虫的分泌蛋白在宿主细胞核内表现出不同的空间和功能活性。然而,这些蛋白质的核定位信号(NLS)在很大程度上仍未得到表征。在这项研究中,我们鉴定并表征了恶性疟原虫(Pf- CSP)的环子孢子蛋白中的两个 NLS 。 Pf -CSP中的两个 NLS 均含有赖氨酸和精氨酸残基簇,这些残基对于与 importin-α 的保守色氨酸和天冬酰胺残基发生特异性相互作用至关重要,从而促进Pf -CSP 的核转位。虽然Pf- CSP的两个 NLS独立发挥作用并且对于核定位都至关重要,但Pf- CSP的单个 NLS会导致核定位较弱。这些发现揭示了疟原虫蛋白分泌蛋白的核穿透性机制。
更新日期:2024-02-18
中文翻译:

恶性疟原虫环子孢子蛋白核定位信号的鉴定和表征
疟原虫的分泌蛋白在宿主细胞核内表现出不同的空间和功能活性。然而,这些蛋白质的核定位信号(NLS)在很大程度上仍未得到表征。在这项研究中,我们鉴定并表征了恶性疟原虫(Pf- CSP)的环子孢子蛋白中的两个 NLS 。 Pf -CSP中的两个 NLS 均含有赖氨酸和精氨酸残基簇,这些残基对于与 importin-α 的保守色氨酸和天冬酰胺残基发生特异性相互作用至关重要,从而促进Pf -CSP 的核转位。虽然Pf- CSP的两个 NLS独立发挥作用并且对于核定位都至关重要,但Pf- CSP的单个 NLS会导致核定位较弱。这些发现揭示了疟原虫蛋白分泌蛋白的核穿透性机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号