当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Measurement and Correlation of Liquid–Liquid Equilibrium Data for Ternary Systems Water + C1–C3 Alcohols + Diethyl Adipate at 298.15 K and Atmospheric Pressure
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-02-19 , DOI: 10.1021/acs.jced.3c00708 Zoran V. Simić 1 , Ivona R. Radović 2 , Mirjana Lj. Kijevčanin 2
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-02-19 , DOI: 10.1021/acs.jced.3c00708 Zoran V. Simić 1 , Ivona R. Radović 2 , Mirjana Lj. Kijevčanin 2
Affiliation
|
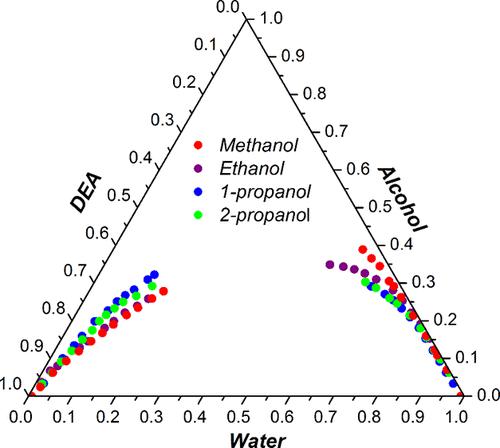
This paper investigates the feasibility of using diethyl adipate (DEA), a green organic solvent, as a separation agent for extracting various alcohols from aqueous solutions. In order to achieve this, liquid–liquid equilibria (LLE) experiments were carried out for four ternary systems: {water + methanol + DEA}, {water + ethanol + DEA}, {water + 1-propanol + DEA}, and {water + 2-propanol + DEA} at 298.15 K and atmospheric pressure. LLE experiments involve determination of certain thermodynamic data such as binodal curves and cloud-point data. Binodal curve data were determined by the cloud-point method using the titration technique, while the tie-line data were determined by optimizing the parameters based on the experimental measurements of the refractive index. Additionally, the distribution coefficients and the separation factors were calculated within the immiscibility region. Hand and Othmer–Tobias correlations were used to examine the reliability of the tie-line data. Also, the experimental ternary LLE data were correlated using the UNIversal QUAsiChemical (UNIQUAC) activity coefficient models. The results confirm that that DEA is a suitable choice for extracting methanol, ethanol, 1-propanol, and 2-propanol from water. The potential of DEA as a separation agent followed the sequence methanol < ethanol < 2-propanol < 1-propanol. UNIQUAC model proved to be effective in correlating the LLE data.
中文翻译:

298.15 K 和大气压下水 + C1–C3 醇 + 己二酸二乙酯三元体系液液平衡数据的测量和关联
本文研究了使用己二酸二乙酯(DEA)这种绿色有机溶剂作为分离剂从水溶液中萃取各种醇的可行性。为了实现这一目标,对四个三元体系进行了液-液平衡(LLE)实验:{水+甲醇+DEA}、{水+乙醇+DEA}、{水+1-丙醇+DEA}和{水 + 2-丙醇 + DEA} 在 298.15 K 和大气压下。LLE 实验涉及确定某些热力学数据,例如双节曲线和浊点数据。双节曲线数据通过使用滴定技术的浊点法确定,而联络线数据通过基于折射率的实验测量优化参数来确定。此外,还计算了不混溶区域内的分配系数和分离因子。使用 Hand 和 Othmer-Tobias 相关性来检验联络线数据的可靠性。此外,实验三元 LLE 数据使用 UNIversal QUAsiChemical (UNIQUAC) 活性系数模型进行关联。结果证实 DEA 是从水中提取甲醇、乙醇、1-丙醇和 2-丙醇的合适选择。DEA 作为分离剂的潜力遵循甲醇 < 乙醇 < 2-丙醇 < 1-丙醇的顺序。UNIQUAC 模型被证明在关联 LLE 数据方面是有效的。
更新日期:2024-02-19
中文翻译:

298.15 K 和大气压下水 + C1–C3 醇 + 己二酸二乙酯三元体系液液平衡数据的测量和关联
本文研究了使用己二酸二乙酯(DEA)这种绿色有机溶剂作为分离剂从水溶液中萃取各种醇的可行性。为了实现这一目标,对四个三元体系进行了液-液平衡(LLE)实验:{水+甲醇+DEA}、{水+乙醇+DEA}、{水+1-丙醇+DEA}和{水 + 2-丙醇 + DEA} 在 298.15 K 和大气压下。LLE 实验涉及确定某些热力学数据,例如双节曲线和浊点数据。双节曲线数据通过使用滴定技术的浊点法确定,而联络线数据通过基于折射率的实验测量优化参数来确定。此外,还计算了不混溶区域内的分配系数和分离因子。使用 Hand 和 Othmer-Tobias 相关性来检验联络线数据的可靠性。此外,实验三元 LLE 数据使用 UNIversal QUAsiChemical (UNIQUAC) 活性系数模型进行关联。结果证实 DEA 是从水中提取甲醇、乙醇、1-丙醇和 2-丙醇的合适选择。DEA 作为分离剂的潜力遵循甲醇 < 乙醇 < 2-丙醇 < 1-丙醇的顺序。UNIQUAC 模型被证明在关联 LLE 数据方面是有效的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号