Synthesis ( IF 2.6 ) Pub Date : 2024-02-20 , DOI: 10.1055/a-2254-0907 Zhaowen Liu 1 , Xiaohua Guo 1 , Zhixi Chen 1 , Longhui Wu 1 , Kai Yang 2
|
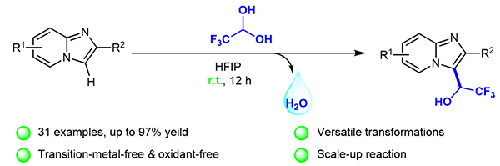
A facile and efficient method for the synthesis of trifluoromethylated carbinols has been developed from imidazo[1,2-a]pyridines and trifluoroacetaldehyde. The direct C(sp2)–H hydroxytrifluoromethylation is successfully implemented at room temperature using HFIP as solvent through dehydrative cross-coupling process, which displays a broad substrate scope and functional group tolerance. Furthermore, gram-scale and synthetic transformation experiments have also been demonstrated, which indicate its potential applicable values in organic synthesis. This green protocol features operational simplicity, atom economy, mild reaction conditions (e.g., at room temperature, transition-metal- and oxidant-free, without inert gas protection), wide substrate scope, and excellent practicality.
中文翻译:

通过咪唑并[1,2-a]吡啶与三氟乙醛脱水偶联无金属合成含三氟甲基甲醇的咪唑并[1,2-a]吡啶
由咪唑并[1,2- a ]吡啶和三氟乙醛开发了一种简便有效的合成三氟甲基化甲醇的方法。以HFIP为溶剂,通过脱水交叉偶联过程在室温下成功实现了直接C(sp 2 )–H羟基三氟甲基化,显示出广泛的底物范围和官能团耐受性。此外,克级和合成转化实验也已得到证实,这表明其在有机合成中的潜在应用价值。该方案具有操作简单、原子经济、反应条件温和(室温、无过渡金属和氧化剂、无需惰性气体保护)、底物适用范围广、实用性高等特点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号