当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asparagine-85 Stabilizes a Structural Active Site Water Network in CYP121A1 of Mycobacterium tuberculosis
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-21 , DOI: 10.1021/acs.biochem.3c00555 Christopher S. Campomizzi 1 , Patil Pranita Uttamrao 2 , Jack J. Stallone 1 , Thenmalarchelvi Rathinavelan 2 , D. Fernando Estrada 1
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-21 , DOI: 10.1021/acs.biochem.3c00555 Christopher S. Campomizzi 1 , Patil Pranita Uttamrao 2 , Jack J. Stallone 1 , Thenmalarchelvi Rathinavelan 2 , D. Fernando Estrada 1
Affiliation
|
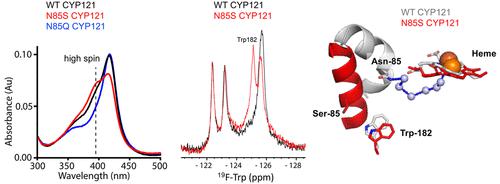
The cytochrome P450 enzyme CYP121A1 endogenously catalyzes the formation of a carbon–carbon bond between the two phenol groups of dicyclotyrosine (cYY) in Mycobacterium tuberculosis (Mtb). One of 20 CYP enzymes in Mtb, CYP121A1 continues to garner significant interest as a potential drug target. The accompanying reports the use of 19F NMR spectroscopy, reconstituted activity assays, and molecular dynamics simulations to investigate the significance of hydrogen bonding interactions that were theorized to stabilize a static active site water network. The active site residue Asn-85, whose hydrogen bonds with the diketopiperazine ring of cYY contributes to a contiguous active site water network in the absence of cYY, was mutated to a serine (N85S) and to a glutamine (N85Q). These conservative changes in the hydrogen bond donor side chain result in inactivation of the enzyme. Moreover, the N85S mutation induces reverse type-I binding as measured by absorbance difference spectra. NMR spectra monitoring the ligand-adaptive FG-loop and the active site Trp-182 side chain confirm that disruption of the active site water network also significantly alters the structure of the active site. These data were consistent with dynamics simulations of N85S and N85Q that demonstrate that a compromised water network is responsible for remodeling of the active site B-helix and a repositioning of cYY toward the heme. These findings implicate a slowly exchanging water network as a critical factor in CYP121A1 function and a likely contributor to the unusual rigidity of the structure.
中文翻译:

Asparagine-85 稳定结核分枝杆菌 CYP121A1 的结构活性位点水网络
结核分枝杆菌(Mtb)中的细胞色素 P450 酶 CYP121A1 内源性催化二环酪氨酸 (cYY) 的两个酚基之间形成碳-碳键。CYP121A1 是 Mtb 中的 20 种 CYP 酶之一,作为潜在的药物靶点继续引起人们的极大兴趣。随附报告使用19 F NMR 光谱、重构活性测定和分子动力学模拟来研究氢键相互作用的重要性,该相互作用理论上可稳定静态活性位点水网络。活性位点残基 Asn-85 突变为丝氨酸 (N85S) 和谷氨酰胺 (N85Q),其与 cYY 的二酮哌嗪环的氢键有助于在不存在 cYY 的情况下形成连续的活性位点水网络。氢键供体侧链的这些保守变化导致酶失活。此外,通过吸光差光谱测量,N85S 突变诱导反向 I 型结合。监测配体自适应 FG 环和活性位点 Trp-182 侧链的 NMR 谱证实,活性位点水网络的破坏也会显着改变活性位点的结构。这些数据与 N85S 和 N85Q 的动力学模拟一致,表明受损的水网络负责活性位点 B 螺旋的重塑以及 cYY 向血红素的重新定位。这些发现表明缓慢交换的水网络是 CYP121A1 功能的关键因素,并且可能是导致结构异常刚性的一个因素。
更新日期:2024-02-21
中文翻译:

Asparagine-85 稳定结核分枝杆菌 CYP121A1 的结构活性位点水网络
结核分枝杆菌(Mtb)中的细胞色素 P450 酶 CYP121A1 内源性催化二环酪氨酸 (cYY) 的两个酚基之间形成碳-碳键。CYP121A1 是 Mtb 中的 20 种 CYP 酶之一,作为潜在的药物靶点继续引起人们的极大兴趣。随附报告使用19 F NMR 光谱、重构活性测定和分子动力学模拟来研究氢键相互作用的重要性,该相互作用理论上可稳定静态活性位点水网络。活性位点残基 Asn-85 突变为丝氨酸 (N85S) 和谷氨酰胺 (N85Q),其与 cYY 的二酮哌嗪环的氢键有助于在不存在 cYY 的情况下形成连续的活性位点水网络。氢键供体侧链的这些保守变化导致酶失活。此外,通过吸光差光谱测量,N85S 突变诱导反向 I 型结合。监测配体自适应 FG 环和活性位点 Trp-182 侧链的 NMR 谱证实,活性位点水网络的破坏也会显着改变活性位点的结构。这些数据与 N85S 和 N85Q 的动力学模拟一致,表明受损的水网络负责活性位点 B 螺旋的重塑以及 cYY 向血红素的重新定位。这些发现表明缓慢交换的水网络是 CYP121A1 功能的关键因素,并且可能是导致结构异常刚性的一个因素。



























 京公网安备 11010802027423号
京公网安备 11010802027423号