当前位置:
X-MOL 学术
›
Forensic Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Forensic implications of novel synthesis of cathinone derivatives by Neber and modified Neber rearrangements
Forensic Chemistry ( IF 2.7 ) Pub Date : 2024-02-17 , DOI: 10.1016/j.forc.2024.100558 Cohan Huxley , Timothy J. Biddle , Ebony Shand , Wendy A. Loughlin , Sarah L. Cresswell , Urs D. Wermuth , Sue E. Boyd , Mark J. Coster
Forensic Chemistry ( IF 2.7 ) Pub Date : 2024-02-17 , DOI: 10.1016/j.forc.2024.100558 Cohan Huxley , Timothy J. Biddle , Ebony Shand , Wendy A. Loughlin , Sarah L. Cresswell , Urs D. Wermuth , Sue E. Boyd , Mark J. Coster
|
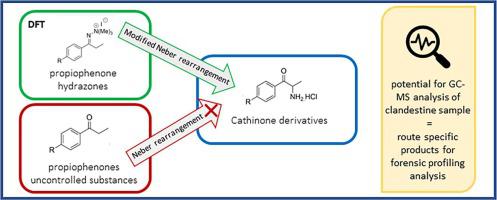
Cathinone and its synthetic analogues are known compounds of clandestine interest. Investigation into novel pathways for synthesising cathinone derivatives has potential for forensic analysis and tracking. The known Neber rearrangement of commercially available phenylpropanones that were evaluated yielded amides described herein and was not viable for clandestine synthesis of cathinone derivatives. Whereas the known modified Neber rearrangement of phenylpropanones that were evaluated via stable aziridine salts and subsequent treatment with acid, gave cathinone derivatives described herein in poor to low yields (2–17%). Assessment of the reagents, equipment, and procedures required for the modified Neber rearrangement was considered as only viable for more advanced clandestine operations. An improved understanding of the potential by-product formation from the modified Neber rearrangement was determined by density functional theory (DFT) of hydrazone to azirine to aziridine intermediates and attempted dynamic NMR spectroscopy of a hydrazone described herein. The substantially lower energy of the azirine step compared to the starting hydrazonium salt step of the reaction mechanism implied that the azirine structure was a short-lived intermediate, and unable to be experimentally determined. New mass-spectral fragmentation data of compounds described herein was reported, where differentiation was observed for some individual compounds at the GC-EIMS fragmentation pattern level. From this study, individual mass-spectrometry fragmentation of key compounds evaluated from the modified Neber rearrangement of commercially available phenylpropanones indicates potential for forensic profiling analysis applications.
中文翻译:

内伯和改良内伯重排新合成卡西酮衍生物的法医学意义
卡西酮及其合成类似物是已知的秘密化合物。对合成卡西酮衍生物的新途径的研究具有法医分析和追踪的潜力。经评估的市售苯丙酮的已知内伯重排产生本文所述的酰胺,并且对于卡西酮衍生物的秘密合成是不可行的。然而,通过稳定的氮丙啶盐和随后用酸处理评估的已知的苯基丙酮的改进的尼伯重排,产生了本文所述的卡西酮衍生物,其产率很差甚至很低(2-17%)。对修改后的内伯重排所需的试剂、设备和程序的评估被认为仅适用于更先进的秘密行动。通过腙至氮丙啶至氮丙啶中间体的密度泛函理论(DFT)并尝试本文所述的腙的动态NMR光谱确定了对由改进的Neber重排形成的潜在副产物的更好的理解。与反应机理的起始腙盐步骤相比,氮丙啶步骤的能量显着降低,这意味着氮丙啶结构是短暂的中间体,并且无法通过实验确定。报道了本文所述的化合物的新的质谱碎片数据,其中在GC-EIMS碎片模式水平上观察到一些单独化合物的差异。根据这项研究,通过市售苯丙酮的改良内伯重排评估的关键化合物的单个质谱碎片表明了法医分析分析应用的潜力。
更新日期:2024-02-17
中文翻译:

内伯和改良内伯重排新合成卡西酮衍生物的法医学意义
卡西酮及其合成类似物是已知的秘密化合物。对合成卡西酮衍生物的新途径的研究具有法医分析和追踪的潜力。经评估的市售苯丙酮的已知内伯重排产生本文所述的酰胺,并且对于卡西酮衍生物的秘密合成是不可行的。然而,通过稳定的氮丙啶盐和随后用酸处理评估的已知的苯基丙酮的改进的尼伯重排,产生了本文所述的卡西酮衍生物,其产率很差甚至很低(2-17%)。对修改后的内伯重排所需的试剂、设备和程序的评估被认为仅适用于更先进的秘密行动。通过腙至氮丙啶至氮丙啶中间体的密度泛函理论(DFT)并尝试本文所述的腙的动态NMR光谱确定了对由改进的Neber重排形成的潜在副产物的更好的理解。与反应机理的起始腙盐步骤相比,氮丙啶步骤的能量显着降低,这意味着氮丙啶结构是短暂的中间体,并且无法通过实验确定。报道了本文所述的化合物的新的质谱碎片数据,其中在GC-EIMS碎片模式水平上观察到一些单独化合物的差异。根据这项研究,通过市售苯丙酮的改良内伯重排评估的关键化合物的单个质谱碎片表明了法医分析分析应用的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号