当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Spiro[isoxazolidino[4,5-f]indolizidine-8,3′-oxindole], Spiro[indolizidine-1,3′-oxindoles], Indolo[2,3-a]quinolizidines and their anti-α-glucosidase activity
Tetrahedron ( IF 2.1 ) Pub Date : 2024-02-20 , DOI: 10.1016/j.tet.2024.133907 Punlop Kuntiyong , Artid Buaphan , Jitnapa Sirirak , Sasipa Booranamonthol , Phongsathon Khlongkhlaeo , Kittisak Thammapichai , Sucharat Sanongkiet
Tetrahedron ( IF 2.1 ) Pub Date : 2024-02-20 , DOI: 10.1016/j.tet.2024.133907 Punlop Kuntiyong , Artid Buaphan , Jitnapa Sirirak , Sasipa Booranamonthol , Phongsathon Khlongkhlaeo , Kittisak Thammapichai , Sucharat Sanongkiet
|
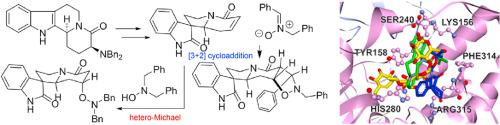
In diastereoselective synthesis of indolo [2,3-]quinolizidine and spiro [indolizidine-1,3′-oxindole] compounds from -glutamic acid, the dibenzylamino group was used for stereocontrol. Its subsequent removal generated unsaturated lactam moiety which provided the point for further derivatization. In particular, during Cope elimination of the dibenzylamino group the by-product -dibenzylhydroxylamine was oxidized to give the corresponding nitrone which underwent tandem [3 + 2]-cycloaddition to give spiro [isoxazolidino [4,5-]indolizidine-8,3′-oxindole] as a single diastereomer. In addition, hetero-Michael addition of the unsaturated lactam with -dibenzylhydroxylamine and methanol gave additional derivatives. All synthetic compounds were tested for their anti-α-glucosidase activity where an indoloquinolizidine and a spiro [indolizidine-1,3′-oxindole] were found to be active against α-glucosidase with IC of 18.06 μM and 13.83 μM, respectively. Molecular docking experiments of selected compounds were conducted to gain an insight into their action and showed that our most active compounds bind to the same site as the positive control, acarbose.
中文翻译:

螺[异恶唑烷基[4,5-f]吲哚里西啶-8,3'-羟吲哚]、螺[吲哚里西啶-1,3'-羟基吲哚]、吲哚并[2,3-a]喹里西啶及其抗α-葡萄糖苷酶的合成活动
在从-谷氨酸非对映选择性合成吲哚并[2,3-]喹里西啶和螺[吲哚里西啶-1,3'-羟吲哚]化合物时,二苄氨基用于立体控制。随后将其除去,生成不饱和内酰胺部分,为进一步衍生化提供了基础。特别是,在Cope消除二苄基氨基的过程中,副产物-二苄基羟胺被氧化,得到相应的硝酮,硝酮进行串联[3 + 2]-环加成,得到螺[异恶唑烷基[4,5-]吲哚里西啶-8,3' -oxindole]作为单一非对映异构体。此外,不饱和内酰胺与二苄基羟胺和甲醇的杂迈克尔加成得到另外的衍生物。测试了所有合成化合物的抗 α-葡萄糖苷酶活性,其中吲哚喹啉西啶和螺环 [indolizidine-1,3'-oxindole] 被发现具有抗 α-葡萄糖苷酶活性,IC 值分别为 18.06 μM 和 13.83 μM。对选定化合物进行了分子对接实验,以深入了解它们的作用,并表明我们最活跃的化合物与阳性对照阿卡波糖结合到同一位点。
更新日期:2024-02-20
中文翻译:

螺[异恶唑烷基[4,5-f]吲哚里西啶-8,3'-羟吲哚]、螺[吲哚里西啶-1,3'-羟基吲哚]、吲哚并[2,3-a]喹里西啶及其抗α-葡萄糖苷酶的合成活动
在从-谷氨酸非对映选择性合成吲哚并[2,3-]喹里西啶和螺[吲哚里西啶-1,3'-羟吲哚]化合物时,二苄氨基用于立体控制。随后将其除去,生成不饱和内酰胺部分,为进一步衍生化提供了基础。特别是,在Cope消除二苄基氨基的过程中,副产物-二苄基羟胺被氧化,得到相应的硝酮,硝酮进行串联[3 + 2]-环加成,得到螺[异恶唑烷基[4,5-]吲哚里西啶-8,3' -oxindole]作为单一非对映异构体。此外,不饱和内酰胺与二苄基羟胺和甲醇的杂迈克尔加成得到另外的衍生物。测试了所有合成化合物的抗 α-葡萄糖苷酶活性,其中吲哚喹啉西啶和螺环 [indolizidine-1,3'-oxindole] 被发现具有抗 α-葡萄糖苷酶活性,IC 值分别为 18.06 μM 和 13.83 μM。对选定化合物进行了分子对接实验,以深入了解它们的作用,并表明我们最活跃的化合物与阳性对照阿卡波糖结合到同一位点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号