当前位置:
X-MOL 学术
›
J. Thorac. Cardiovasc. Surg.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
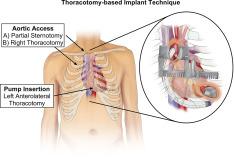
Ventricular assist device using a thoracotomy-based implant technique: Multi-Center Implantation of the HeartMate 3 in Subjects With Heart Failure Using Surgical Techniques Other Than Full Median Sternotomy (HM3 SWIFT)
The Journal of Thoracic and Cardiovascular Surgery ( IF 6 ) Pub Date : 2024-02-15 , DOI: 10.1016/j.jtcvs.2024.02.013 Igor Gosev , Duc Thinh Pham , John Y. Um , Anelechi C. Anyanwu , Akinobu Itoh , Kunal Kotkar , Koji Takeda , Yoshifumi Naka , Matthias Peltz , Scott C. Silvestry , Gregory Couper , Marzia Leacche , Vivek Rao , Benjamin Sun , Ryan J. Tedford , Nahush Mokadam , Robert McNutt , Daniel Crandall , Mandeep R. Mehra , Christopher T. Salerno
The Journal of Thoracic and Cardiovascular Surgery ( IF 6 ) Pub Date : 2024-02-15 , DOI: 10.1016/j.jtcvs.2024.02.013 Igor Gosev , Duc Thinh Pham , John Y. Um , Anelechi C. Anyanwu , Akinobu Itoh , Kunal Kotkar , Koji Takeda , Yoshifumi Naka , Matthias Peltz , Scott C. Silvestry , Gregory Couper , Marzia Leacche , Vivek Rao , Benjamin Sun , Ryan J. Tedford , Nahush Mokadam , Robert McNutt , Daniel Crandall , Mandeep R. Mehra , Christopher T. Salerno
|
The HeartMate 3 (Abbott) left ventricular assist device provides substantial improvement in long-term morbidity and mortality in patients with advanced heart failure. The Implantation of the HeartMate 3 in Subjects With Heart Failure Using Surgical Techniques Other Than Full Median Sternotomy study compares thoracotomy-based implantation clinical outcomes with standard median sternotomy. We conducted a prospective, multicenter, single-arm study in patients eligible for HeartMate 3 implantation with thoracotomy-based surgical technique (bilateral thoracotomy or partial upper sternotomy with left thoracotomy). The composite primary end point was survival free of disabling stroke (modified Rankin score >3), or reoperation to remove or replace a malfunctioning device, or conversion to median sternotomy at 6-months postimplant (elective transplants were treated as a success). The primary end point (noninferiority, −15% margin) was assessed with >90% power compared with a propensity score-matched cohort (ratio 1:2) derived from the Multi-Center Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3 continued access protocol. The study enrolled 102 patients between December 2020 and July 2022 in the thoracotomy-based arm at 23 North American centers. Follow-up concluded in December 2022. In the Implantation of the HeartMate 3 in Subjects With Heart Failure Using Surgical Techniques Other Than Full Median Sternotomy study group, noninferiority criteria was met (absolute between-group difference, −1.2%; Farrington Manning lower 1-sided 95% CI, −9.3%; < .0025) and event-free survival was not different (85.0% vs 86.2%; hazard ratio, 1.01; 95% CI, 0.58-2.10). Length of stay with thoracotomy-based implant was longer (median, 20 vs 17 days; = .03). No differences were observed for blood product utilization, adverse events (including right heart failure), functional status, and quality of life between cohorts. Thoracotomy-based implantation of the HeartMate 3 left ventricular assist device is noninferior to implantation via standard full sternotomy. This study supports thoracotomy-based implantation as an additional standard for surgical implantation of the HeartMate 3 left ventricular assist device.
中文翻译:

使用基于胸廓切开术的植入技术的心室辅助装置:使用全正中胸骨切开术以外的手术技术在心力衰竭受试者中多中心植入 HeartMate 3 (HM3 SWIFT)
HeartMate 3 (Abbott) 左心室辅助装置可显着改善晚期心力衰竭患者的长期发病率和死亡率。使用除全中位胸骨切开术以外的手术技术在心力衰竭受试者中植入 HeartMate 3 研究比较了基于开胸术的植入临床结果与标准中位胸骨切开术。我们对符合开胸手术技术(双侧开胸术或部分上胸骨切开术和左开胸术)植入 HeartMate 3 的患者进行了一项前瞻性、多中心、单臂研究。复合主要终点是无致残性中风的存活率(改良Rankin评分>3),或再次手术移除或更换故障装置,或在植入后6个月转为正中胸骨切开术(选择性移植被视为成功)。与倾向评分匹配队列(比例 1:2)相比,主要终点(非劣效性,-15% 裕度)的功效评估大于 90%,该队列源自 MagLev 技术在接受机械循环支持治疗的患者中的多中心研究使用 HeartMate 3 继续访问协议。该研究在 2020 年 12 月至 2022 年 7 月期间在 23 个北美中心的开胸手术组中招募了 102 名患者。随访于 2022 年 12 月结束。在使用全中位胸骨切开术以外的手术技术对心力衰竭受试者植入 HeartMate 3 的研究组中,满足非劣效性标准(组间绝对差异为 -1.2%;Farrington Manning 较低 1)单侧 95% CI,-9.3%;< .0025)和无事件生存期没有差异(85.0% vs 86.2%;风险比,1.01;95% CI,0.58-2.10)。开胸植入术的住院时间更长(中位时间为 20 天 vs 17 天;= .03)。各队列之间的血液制品利用率、不良事件(包括右心衰竭)、功能状态和生活质量没有观察到差异。通过开胸术植入 HeartMate 3 左心室辅助装置并不劣于通过标准全胸骨切开术植入。本研究支持开胸植入作为 HeartMate 3 左心室辅助装置手术植入的附加标准。
更新日期:2024-02-15
中文翻译:

使用基于胸廓切开术的植入技术的心室辅助装置:使用全正中胸骨切开术以外的手术技术在心力衰竭受试者中多中心植入 HeartMate 3 (HM3 SWIFT)
HeartMate 3 (Abbott) 左心室辅助装置可显着改善晚期心力衰竭患者的长期发病率和死亡率。使用除全中位胸骨切开术以外的手术技术在心力衰竭受试者中植入 HeartMate 3 研究比较了基于开胸术的植入临床结果与标准中位胸骨切开术。我们对符合开胸手术技术(双侧开胸术或部分上胸骨切开术和左开胸术)植入 HeartMate 3 的患者进行了一项前瞻性、多中心、单臂研究。复合主要终点是无致残性中风的存活率(改良Rankin评分>3),或再次手术移除或更换故障装置,或在植入后6个月转为正中胸骨切开术(选择性移植被视为成功)。与倾向评分匹配队列(比例 1:2)相比,主要终点(非劣效性,-15% 裕度)的功效评估大于 90%,该队列源自 MagLev 技术在接受机械循环支持治疗的患者中的多中心研究使用 HeartMate 3 继续访问协议。该研究在 2020 年 12 月至 2022 年 7 月期间在 23 个北美中心的开胸手术组中招募了 102 名患者。随访于 2022 年 12 月结束。在使用全中位胸骨切开术以外的手术技术对心力衰竭受试者植入 HeartMate 3 的研究组中,满足非劣效性标准(组间绝对差异为 -1.2%;Farrington Manning 较低 1)单侧 95% CI,-9.3%;< .0025)和无事件生存期没有差异(85.0% vs 86.2%;风险比,1.01;95% CI,0.58-2.10)。开胸植入术的住院时间更长(中位时间为 20 天 vs 17 天;= .03)。各队列之间的血液制品利用率、不良事件(包括右心衰竭)、功能状态和生活质量没有观察到差异。通过开胸术植入 HeartMate 3 左心室辅助装置并不劣于通过标准全胸骨切开术植入。本研究支持开胸植入作为 HeartMate 3 左心室辅助装置手术植入的附加标准。



























 京公网安备 11010802027423号
京公网安备 11010802027423号