当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Integration of Microfluidic Devices with Microelectrode Arrays to Functionally Assay Amyloid-β-Induced Synaptotoxicity
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2024-02-22 , DOI: 10.1021/acsbiomaterials.3c00997 Camille Lefebvre 1 , Anaïs-Camille Vreulx 2 , Corentin Dumortier 1 , Séverine Bégard 1 , Carla Gelle 2 , Dolores Siedlecki-Wullich 2 , Morvane Colin 1 , Devrim Kilinc 2 , Sophie Halliez 1
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2024-02-22 , DOI: 10.1021/acsbiomaterials.3c00997 Camille Lefebvre 1 , Anaïs-Camille Vreulx 2 , Corentin Dumortier 1 , Séverine Bégard 1 , Carla Gelle 2 , Dolores Siedlecki-Wullich 2 , Morvane Colin 1 , Devrim Kilinc 2 , Sophie Halliez 1
Affiliation
|
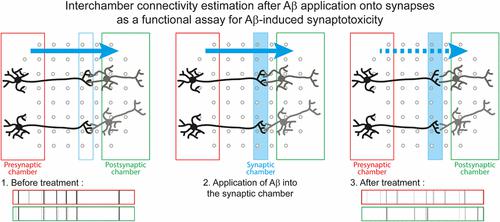
Alzheimer’s disease (AD) is a neurodegenerative disease and the most frequent cause of dementia. It is characterized by the accumulation in the brain of two pathological protein aggregates: amyloid-β peptides (Aβ) and abnormally phosphorylated tau. The progressive cognitive decline observed in patients strongly correlates with the synaptic loss. Many lines of evidence suggest that soluble forms of Aβ accumulate into the brain where they cause synapse degeneration. Stopping their spreading and/or targeting the pathophysiological mechanisms leading to synaptic loss would logically be beneficial for the patients. However, we are still far from understanding these processes. Our objective was therefore to develop a versatile model to assay and study Aβ-induced synaptotoxicity. We integrated a microfluidic device that physically isolates synapses from presynaptic and postsynaptic neurons with a microelectrode array. We seeded mouse primary cortical cells in the presynaptic and postsynaptic chambers. After functional synapses have formed in the synaptic chamber, we exposed them to concentrated conditioned media from cell lines overexpressing the wild-type or mutated amyloid precursor protein and thus secreting different levels of Aβ. We recorded the neuronal activity before and after exposition to Aβ and quantified Aβ’s effects on the connectivity between presynaptic and postsynaptic neurons. We observed that the application of Aβ on the synapses for 48 h strongly decreased the interchamber connectivity without significantly affecting the neuronal activity in the presynaptic or postsynaptic chambers. Thus, through this model, we are able to functionally assay the impact of Aβ peptides (or other molecules) on synaptic connectivity and to use the latter as a proxy to study Aβ-induced synaptotoxicity. Moreover, since the presynaptic, postsynaptic, and synaptic chambers can be individually targeted, our assay provides a powerful tool to evaluate the involvement of candidate genes in synaptic vulnerability and/or test therapeutic strategies for AD.
中文翻译:

微流体装置与微电极阵列的集成对淀粉样蛋白-β-诱导的突触毒性进行功能测定
阿尔茨海默病(AD)是一种神经退行性疾病,也是痴呆症最常见的原因。其特征是两种病理性蛋白质聚集体在大脑中积聚:淀粉样蛋白-β 肽 (Aβ) 和异常磷酸化的 tau 蛋白。在患者中观察到的进行性认知能力下降与突触丧失密切相关。许多证据表明,可溶形式的 Aβ 会积聚到大脑中,导致突触退化。阻止它们的扩散和/或针对导致突触丧失的病理生理机制在逻辑上对患者是有益的。然而,我们距离理解这些过程还很远。因此,我们的目标是开发一种通用模型来测定和研究 Aβ 诱导的突触毒性。我们集成了一种微流体装置,该装置通过微电极阵列将突触与突触前和突触后神经元物理隔离。我们将小鼠原代皮质细胞接种在突触前室和突触后室中。在突触室中形成功能性突触后,我们将它们暴露于来自过表达野生型或突变淀粉样前体蛋白的细胞系的浓缩条件培养基中,从而分泌不同水平的 Aβ。我们记录了暴露于 Aβ 之前和之后的神经元活动,并量化了 Aβ 对突触前和突触后神经元之间连接的影响。我们观察到,在突触上应用 Aβ 48 小时,会强烈降低室间连接,但不会显着影响突触前或突触后室的神经元活动。因此,通过这个模型,我们能够在功能上分析 Aβ 肽(或其他分子)对突触连接的影响,并使用后者作为研究 Aβ 诱导的突触毒性的代理。此外,由于突触前、突触后和突触室可以单独靶向,因此我们的测定提供了一个强大的工具来评估候选基因在突触脆弱性中的参与和/或测试 AD 治疗策略。
更新日期:2024-02-22
中文翻译:

微流体装置与微电极阵列的集成对淀粉样蛋白-β-诱导的突触毒性进行功能测定
阿尔茨海默病(AD)是一种神经退行性疾病,也是痴呆症最常见的原因。其特征是两种病理性蛋白质聚集体在大脑中积聚:淀粉样蛋白-β 肽 (Aβ) 和异常磷酸化的 tau 蛋白。在患者中观察到的进行性认知能力下降与突触丧失密切相关。许多证据表明,可溶形式的 Aβ 会积聚到大脑中,导致突触退化。阻止它们的扩散和/或针对导致突触丧失的病理生理机制在逻辑上对患者是有益的。然而,我们距离理解这些过程还很远。因此,我们的目标是开发一种通用模型来测定和研究 Aβ 诱导的突触毒性。我们集成了一种微流体装置,该装置通过微电极阵列将突触与突触前和突触后神经元物理隔离。我们将小鼠原代皮质细胞接种在突触前室和突触后室中。在突触室中形成功能性突触后,我们将它们暴露于来自过表达野生型或突变淀粉样前体蛋白的细胞系的浓缩条件培养基中,从而分泌不同水平的 Aβ。我们记录了暴露于 Aβ 之前和之后的神经元活动,并量化了 Aβ 对突触前和突触后神经元之间连接的影响。我们观察到,在突触上应用 Aβ 48 小时,会强烈降低室间连接,但不会显着影响突触前或突触后室的神经元活动。因此,通过这个模型,我们能够在功能上分析 Aβ 肽(或其他分子)对突触连接的影响,并使用后者作为研究 Aβ 诱导的突触毒性的代理。此外,由于突触前、突触后和突触室可以单独靶向,因此我们的测定提供了一个强大的工具来评估候选基因在突触脆弱性中的参与和/或测试 AD 治疗策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号