当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterization of the Zinc Uptake Repressor (Zur) from Acinetobacter baumannii
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-22 , DOI: 10.1021/acs.biochem.3c00679 Minyong Kim 1, 2 , My Tra Le 2 , Lixin Fan 3 , Courtney Campbell 4 , Sambuddha Sen 1 , Daiana A. Capdevila 5 , Timothy L. Stemmler 4 , David P. Giedroc 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-22 , DOI: 10.1021/acs.biochem.3c00679 Minyong Kim 1, 2 , My Tra Le 2 , Lixin Fan 3 , Courtney Campbell 4 , Sambuddha Sen 1 , Daiana A. Capdevila 5 , Timothy L. Stemmler 4 , David P. Giedroc 1, 2
Affiliation
|
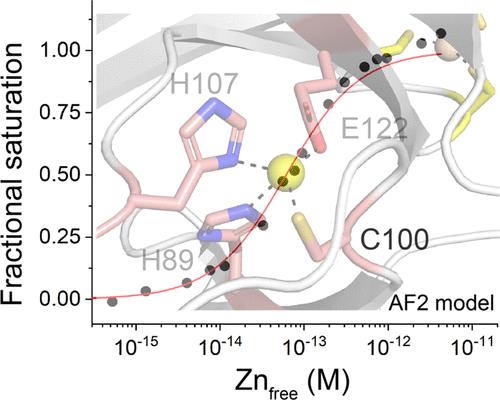
Bacterial cells tightly regulate the intracellular concentrations of essential transition metal ions by deploying a panel of metal-regulated transcriptional repressors and activators that bind to operator-promoter regions upstream of regulated genes. Like other zinc uptake regulator (Zur) proteins, Acinetobacter baumannii Zur represses transcription of its regulon when ZnII is replete and binds more weakly to DNA when ZnII is limiting. Previous studies established that Zur proteins are homodimeric and harbor at least two metal sites per protomer or four per dimer. CdII X-ray absorption spectroscopy (XAS) of the Cd2Zn2 AbZur metalloderivative with CdII bound to the allosteric sites reveals a S(N/O)3 first coordination shell. Site-directed mutagenesis suggests that H89 and C100 from the N-terminal DNA binding domain and H107 and E122 from the C-terminal dimerization domain comprise the regulatory metal site. KZn for this allosteric site is 6.0 (±2.2) × 1012 M–1 with a functional “division of labor” among the four metal ligands. N-terminal domain ligands H89 and C100 contribute far more to KZn than H107 and E122, while C100S AbZur uniquely fails to bind to DNA tightly as measured by an in vitro transcription assay. The heterotropic allosteric coupling free energy, ΔGc, is negative, consistent with a higher KZn for the AbZur-DNA complex and defining a bioavailable ZnII set-point of ≈6 × 10–14 M. Small-angle X-ray scattering (SAXS) experiments reveal that only the wild-type Zn homodimer undergoes allosteric switching, while the C100S AbZur fails to switch. These data collectively suggest that switching to a high affinity DNA-binding conformation involves a rotation/translation of one protomer relative to the other in a way that is dependent on the integrity of C100. We place these findings in the context of other Zur proteins and Fur family repressors more broadly.
中文翻译:

鲍曼不动杆菌锌摄取阻遏物 (Zur) 的表征
细菌细胞通过部署一组金属调节的转录抑制子和激活子来严格调节必需过渡金属离子的细胞内浓度,这些转录抑制子和激活子与受调节基因上游的操纵子启动子区域结合。与其他锌摄取调节蛋白 (Zur) 一样,鲍曼不动杆菌Zur 在 Zn II充足时抑制其调节子的转录,而在 Zn II限制时与 DNA 的结合更弱。先前的研究表明,Zur 蛋白是同型二聚体,每个原聚体至少有两个金属位点,每个二聚体至少有四个金属位点。Cd II与变构位点结合的 Cd 2 Zn 2 Ab Zur 金属衍生物的 Cd II X 射线吸收光谱 (XAS)显示出 S(N/O) 3第一配位壳。定点诱变表明,来自 N 端 DNA 结合结构域的 H89 和 C100 以及来自 C 端二聚化结构域的 H107 和 E122 构成了调节金属位点。该变构位点的K Zn为 6.0 (±2.2) × 10 12 M –1,在四种金属配体之间具有功能性“分工”。N 端结构域配体 H89 和 C100 对K Zn的贡献远大于 H107 和 E122,而根据体外转录测定的测量,C100S Ab Zur 独特地未能与 DNA 紧密结合。异方变构耦合自由能 Δ G c为负值,与Ab Zur-DNA 复合物的较高K Zn一致,并定义生物可利用的 Zn II设定点 ≈6 × 10 –14 M。小角度 X-射线散射 (SAXS) 实验表明,只有野生型 Zn 同二聚体发生变构转换,而 C100S Ab Zur 则无法转换。这些数据共同表明,转换到高亲和力 DNA 结合构象涉及一个原体相对于另一个原体的旋转/平移,其方式取决于 C100 的完整性。我们将这些发现更广泛地置于其他 Zur 蛋白和 Fur 家族阻遏蛋白的背景下。
更新日期:2024-02-22
中文翻译:

鲍曼不动杆菌锌摄取阻遏物 (Zur) 的表征
细菌细胞通过部署一组金属调节的转录抑制子和激活子来严格调节必需过渡金属离子的细胞内浓度,这些转录抑制子和激活子与受调节基因上游的操纵子启动子区域结合。与其他锌摄取调节蛋白 (Zur) 一样,鲍曼不动杆菌Zur 在 Zn II充足时抑制其调节子的转录,而在 Zn II限制时与 DNA 的结合更弱。先前的研究表明,Zur 蛋白是同型二聚体,每个原聚体至少有两个金属位点,每个二聚体至少有四个金属位点。Cd II与变构位点结合的 Cd 2 Zn 2 Ab Zur 金属衍生物的 Cd II X 射线吸收光谱 (XAS)显示出 S(N/O) 3第一配位壳。定点诱变表明,来自 N 端 DNA 结合结构域的 H89 和 C100 以及来自 C 端二聚化结构域的 H107 和 E122 构成了调节金属位点。该变构位点的K Zn为 6.0 (±2.2) × 10 12 M –1,在四种金属配体之间具有功能性“分工”。N 端结构域配体 H89 和 C100 对K Zn的贡献远大于 H107 和 E122,而根据体外转录测定的测量,C100S Ab Zur 独特地未能与 DNA 紧密结合。异方变构耦合自由能 Δ G c为负值,与Ab Zur-DNA 复合物的较高K Zn一致,并定义生物可利用的 Zn II设定点 ≈6 × 10 –14 M。小角度 X-射线散射 (SAXS) 实验表明,只有野生型 Zn 同二聚体发生变构转换,而 C100S Ab Zur 则无法转换。这些数据共同表明,转换到高亲和力 DNA 结合构象涉及一个原体相对于另一个原体的旋转/平移,其方式取决于 C100 的完整性。我们将这些发现更广泛地置于其他 Zur 蛋白和 Fur 家族阻遏蛋白的背景下。



























 京公网安备 11010802027423号
京公网安备 11010802027423号