当前位置:
X-MOL 学术
›
Chem. Eng. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thermodynamics of Reversible Hydrogen Storage: Are Methoxy-Substituted Aromatics better through Oxygen Functionality?
Chemical Engineering & Technology ( IF 2.1 ) Pub Date : 2024-02-21 , DOI: 10.1002/ceat.202300358 Artemiy A. Samarov 1 , Karsten Müller 2, 3 , Peter Wasserscheid 4, 5 , Sergey P. Verevkin 3, 6
Chemical Engineering & Technology ( IF 2.1 ) Pub Date : 2024-02-21 , DOI: 10.1002/ceat.202300358 Artemiy A. Samarov 1 , Karsten Müller 2, 3 , Peter Wasserscheid 4, 5 , Sergey P. Verevkin 3, 6
Affiliation
|
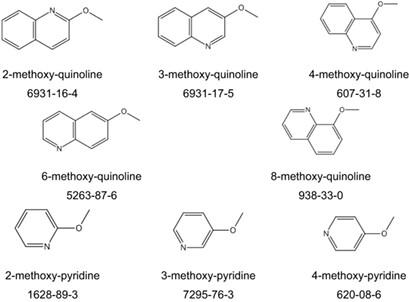
An attractive option for the storage of hydrogen is the reversible hydrogenation of an aromatic substance, a so-called liquid organic hydrogen carrier. For hydrogen release, it is desirable to find substances with low enthalpies of reaction for dehydrogenation. It has been demonstrated that substitution, e.g., with methyl groups, on the ring can have a positive effect in this regard. In this study, thermochemical properties of methoxy-substituted pyridines and quinolines and their respective hydrogenated counterparts have been analyzed from a thermodynamic point of view. Combustion calorimetry has been utilized to measure the enthalpy of formation and the transpiration method for determining the vapor pressure. Additionally, high-level quantum-chemical approaches have been applied to obtain caloric data in the gas phase, which provide a basis for comparison and evaluation of the experimental data. The results reveal two main effects of methoxy substitution. First, oxygenated substituents like methoxy groups have stronger effect on the Gibbs energy of reaction and thus the temperature needed thermodynamically for hydrogen release than methyl groups. Second, the effect is highly depending on the position of the substitution on the ring. Particularly methoxy groups close to a N heteroatom can have a significant positive effect, which might enable hydrogen release at equilibrium temperatures more than 50 K lower than for their unsubstituted analogue.
中文翻译:

可逆储氢的热力学:甲氧基取代的芳烃通过氧官能度更好吗?
氢存储的一个有吸引力的选择是芳香族物质的可逆氢化,即所谓的液态有机氢载体。对于氢释放,需要找到脱氢反应焓低的物质。已经证明,环上的取代,例如用甲基,在这方面可以具有积极的效果。在本研究中,从热力学角度分析了甲氧基取代的吡啶和喹啉及其各自的氢化对应物的热化学性质。燃烧量热法已用于测量生成热,蒸腾法已用于确定蒸气压。此外,高级量子化学方法已应用于获得气相热量数据,为实验数据的比较和评估提供基础。结果揭示了甲氧基取代的两个主要影响。首先,甲氧基等含氧取代基对反应吉布斯能的影响更强,因此对氢释放热力学所需温度的影响比甲基更强。其次,效果很大程度上取决于环上取代的位置。特别是靠近 N 杂原子的甲氧基可以具有显着的积极作用,这可能使氢在平衡温度下释放,比未取代的类似物低 50 K 以上。
更新日期:2024-02-21
中文翻译:

可逆储氢的热力学:甲氧基取代的芳烃通过氧官能度更好吗?
氢存储的一个有吸引力的选择是芳香族物质的可逆氢化,即所谓的液态有机氢载体。对于氢释放,需要找到脱氢反应焓低的物质。已经证明,环上的取代,例如用甲基,在这方面可以具有积极的效果。在本研究中,从热力学角度分析了甲氧基取代的吡啶和喹啉及其各自的氢化对应物的热化学性质。燃烧量热法已用于测量生成热,蒸腾法已用于确定蒸气压。此外,高级量子化学方法已应用于获得气相热量数据,为实验数据的比较和评估提供基础。结果揭示了甲氧基取代的两个主要影响。首先,甲氧基等含氧取代基对反应吉布斯能的影响更强,因此对氢释放热力学所需温度的影响比甲基更强。其次,效果很大程度上取决于环上取代的位置。特别是靠近 N 杂原子的甲氧基可以具有显着的积极作用,这可能使氢在平衡温度下释放,比未取代的类似物低 50 K 以上。



























 京公网安备 11010802027423号
京公网安备 11010802027423号