Cell Chemical Biology ( IF 8.6 ) Pub Date : 2024-02-22 , DOI: 10.1016/j.chembiol.2024.01.010 Emily Puumala , David Sychantha , Elizabeth Lach , Shawn Reeves , Sunna Nabeela , Meea Fogal , AkshatKumar Nigam , Jarrod W. Johnson , Alán Aspuru-Guzik , Rebecca S. Shapiro , Priya Uppuluri , Subha Kalyaanamoorthy , Jakob Magolan , Luke Whitesell , Nicole Robbins , Gerard D. Wright , Leah E. Cowen
|
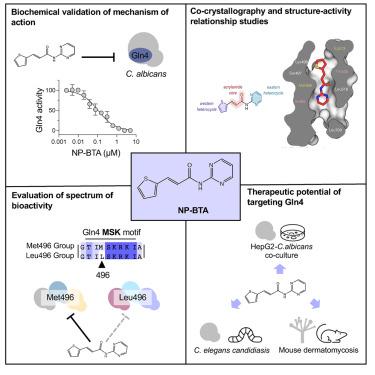
Candida species are among the most prevalent causes of systemic fungal infections, which account for ∼1.5 million annual fatalities. Here, we build on a compound screen that identified the molecule N-pyrimidinyl-β-thiophenylacrylamide (NP-BTA), which strongly inhibits Candida albicans growth. NP-BTA was hypothesized to target C. albicans glutaminyl-tRNA synthetase, Gln4. Here, we confirmed through in vitro amino-acylation assays NP-BTA is a potent inhibitor of Gln4, and we defined how NP-BTA arrests Gln4’s transferase activity using co-crystallography. This analysis also uncovered Met496 as a critical residue for the compound’s species-selective target engagement and potency. Structure-activity relationship (SAR) studies demonstrated the NP-BTA scaffold is subject to oxidative and non-oxidative metabolism, making it unsuitable for systemic administration. In a mouse dermatomycosis model, however, topical application of the compound provided significant therapeutic benefit. This work expands the repertoire of antifungal protein synthesis target mechanisms and provides a path to develop Gln4 inhibitors.
中文翻译:

N-嘧啶基-β-苯硫基丙烯酰胺对 tRNA 合成酶 Gln4 的变构抑制发挥了高度选择性的抗真菌活性
念珠菌属是全身性真菌感染最常见的原因之一,每年导致约 150 万人死亡。在这里,我们建立了一个化合物筛选,鉴定出 N-嘧啶基-β-苯硫基丙烯酰胺 (NP-BTA) 分子,该分子强烈抑制白色念珠菌的生长。假设 NP-BTA 靶向白色念珠菌谷氨酰胺酰-tRNA 合成酶 Gln4。在这里,我们通过体外氨酰化测定证实 NP-BTA 是 Gln4 的有效抑制剂,并使用共结晶术定义了 NP-BTA 如何抑制 Gln4 的转移酶活性。该分析还发现 Met496 是该化合物的物种选择性靶点参与和效力的关键残留物。构效关系(SAR)研究表明,NP-BTA 支架会发生氧化和非氧化代谢,因此不适合全身给药。然而,在小鼠皮肤真菌病模型中,局部应用该化合物提供了显着的治疗效果。这项工作扩展了抗真菌蛋白合成靶点机制的库,并提供了开发 Gln4 抑制剂的途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号