当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, antibacterial activity, and 3D-QASR studies of matrine-indole derivatives as potential antibiotics
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2024-02-21 , DOI: 10.1016/j.bmcl.2024.129671 Yufang Li , Jamal A.H. Kowah , Meiyan Jiang , Yaqing Wu , Lisheng Wang , Fangfang Yang
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2024-02-21 , DOI: 10.1016/j.bmcl.2024.129671 Yufang Li , Jamal A.H. Kowah , Meiyan Jiang , Yaqing Wu , Lisheng Wang , Fangfang Yang
|
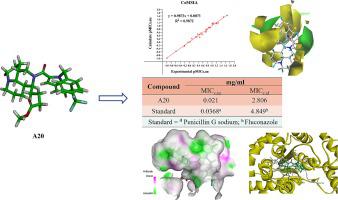
Matrine and indole have antibacterial, anticancer, and other biological activities, in order to develop new antibiotics to solve the problem of multi-drug resistant bacteria. In this paper, we synthesized a series of 29 novel matrine derivatives as potential drug candidates by combining indole analogs and matrine. The antibacterial activity of these compounds was evaluated through minimum inhibitory concentration (MIC) assays against five bacterial strains (, , , , and ). The obtained results demonstrated promising antibacterial efficacy, particularly for compounds and , which exhibited MIC values of 0.021 and 0.031 mg/ml, respectively, against . Moreover, compounds and displayed remarkable MIC values of 2.806 and 4.519 mg/ml, respectively, against , surpassing the performance of the clinical antibiotic penicillin G sodium (0.0368 mg/ml) and fluconazole (4.849 mg/ml). These findings underscore the significant bacteriostatic activity of the matrine derivatives. Furthermore, to gain a deeper understanding 3D-QSAR modeling was employed, revealing the critical influence of steric structure, charge distribution, hydrophobic interactions, and hydrogen bonding within the molecular structure on the bacteriostatic activity of the compounds. Additionally, molecular docking simulations shed light on the interaction between compound and bacterial proteins, highlighting the involvement of hydrogen bonding, hydrophobic interactions, and π-π conjugation in the formation of stable complexes that inhibit the normal functioning of the proteins. This comprehensive analysis provided valuable insights into the antibacterial mechanism of the novel matrine derivatives, offering theoretical support for their potential application as antibiotics.
中文翻译:

作为潜在抗生素的苦参碱吲哚衍生物的合成、抗菌活性和 3D-QASR 研究
苦参碱和吲哚具有抗菌、抗癌等生物活性,以便开发新型抗生素解决细菌多重耐药问题。在本文中,我们通过结合吲哚类似物和苦参碱合成了一系列 29 种新型苦参碱衍生物作为潜在的候选药物。通过针对五种细菌菌株(、、、、和)的最低抑菌浓度(MIC)测定来评估这些化合物的抗菌活性。获得的结果表明具有良好的抗菌功效,尤其是化合物 和 ,其针对 的 MIC 值分别为 0.021 和 0.031 mg/ml。此外,化合物 和 分别表现出显着的 MIC 值,分别为 2.806 和 4.519 mg/ml,超过了临床抗生素青霉素 G 钠 (0.0368 mg/ml) 和氟康唑 (4.849 mg/ml) 的性能。这些发现强调了苦参碱衍生物的显着抑菌活性。此外,为了更深入地了解,采用了 3D-QSAR 模型,揭示了分子结构内的空间结构、电荷分布、疏水相互作用和氢键对化合物抑菌活性的关键影响。此外,分子对接模拟揭示了化合物与细菌蛋白质之间的相互作用,强调了氢键、疏水相互作用和 π-π 共轭在抑制蛋白质正常功能的稳定复合物形成中的参与。这项综合分析为新型苦参碱衍生物的抗菌机制提供了有价值的见解,为其作为抗生素的潜在应用提供了理论支持。
更新日期:2024-02-21
中文翻译:

作为潜在抗生素的苦参碱吲哚衍生物的合成、抗菌活性和 3D-QASR 研究
苦参碱和吲哚具有抗菌、抗癌等生物活性,以便开发新型抗生素解决细菌多重耐药问题。在本文中,我们通过结合吲哚类似物和苦参碱合成了一系列 29 种新型苦参碱衍生物作为潜在的候选药物。通过针对五种细菌菌株(、、、、和)的最低抑菌浓度(MIC)测定来评估这些化合物的抗菌活性。获得的结果表明具有良好的抗菌功效,尤其是化合物 和 ,其针对 的 MIC 值分别为 0.021 和 0.031 mg/ml。此外,化合物 和 分别表现出显着的 MIC 值,分别为 2.806 和 4.519 mg/ml,超过了临床抗生素青霉素 G 钠 (0.0368 mg/ml) 和氟康唑 (4.849 mg/ml) 的性能。这些发现强调了苦参碱衍生物的显着抑菌活性。此外,为了更深入地了解,采用了 3D-QSAR 模型,揭示了分子结构内的空间结构、电荷分布、疏水相互作用和氢键对化合物抑菌活性的关键影响。此外,分子对接模拟揭示了化合物与细菌蛋白质之间的相互作用,强调了氢键、疏水相互作用和 π-π 共轭在抑制蛋白质正常功能的稳定复合物形成中的参与。这项综合分析为新型苦参碱衍生物的抗菌机制提供了有价值的见解,为其作为抗生素的潜在应用提供了理论支持。



























 京公网安备 11010802027423号
京公网安备 11010802027423号