当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Disruption of an Active Site Network Leads to Activation of C2α-Lactylthiamin Diphosphate on the Antibacterial Target 1-Deoxy-d-xylulose-5-phosphate Synthase
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-23 , DOI: 10.1021/acs.biochem.3c00735 Eucolona M. Toci 1 , Steven L. Austin 2 , Ananya Majumdar 3 , H. Lee Woodcock 2 , Caren L. Freel Meyers 1
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-23 , DOI: 10.1021/acs.biochem.3c00735 Eucolona M. Toci 1 , Steven L. Austin 2 , Ananya Majumdar 3 , H. Lee Woodcock 2 , Caren L. Freel Meyers 1
Affiliation
|
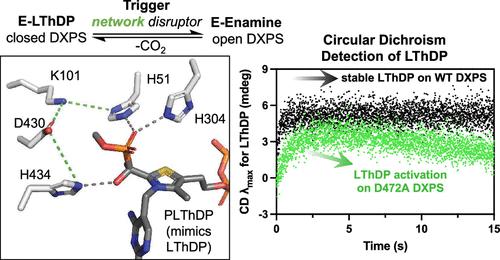
The bacterial metabolic enzyme 1-deoxy-d-xylulose-5-phosphate synthase (DXPS) catalyzes the thiamin diphosphate (ThDP)-dependent formation of DXP from pyruvate and d-glyceraldehyde-3-phosphate (d-GAP). DXP is an essential bacteria-specific metabolite that feeds into the biosynthesis of isoprenoids, pyridoxal phosphate (PLP), and ThDP. DXPS catalyzes the activation of pyruvate to give the C2α-lactylThDP (LThDP) adduct that is long-lived on DXPS in a closed state in the absence of the cosubstrate. Binding of d-GAP shifts the DXPS-LThDP complex to an open state which coincides with LThDP decarboxylation. This gated mechanism distinguishes DXPS in ThDP enzymology. How LThDP persists on DXPS in the absence of cosubstrate, while other pyruvate decarboxylases readily activate LThDP for decarboxylation, is a long-standing question in the field. We propose that an active site network functions to prevent LThDP activation on DXPS until the cosubstrate binds. Binding of d-GAP coincides with a conformational shift and disrupts the network causing changes in the active site that promote LThDP activation. Here, we show that the substitution of putative network residues, as well as nearby residues believed to contribute to network charge distribution, predictably affects LThDP reactivity. Substitutions predicted to disrupt the network have the effect to activate LThDP for decarboxylation, resulting in CO2 and acetate production. In contrast, a substitution predicted to strengthen the network fails to activate LThDP and has the effect to shift DXPS toward the closed state. Network-disrupting substitutions near the carboxylate of LThDP also have a pronounced effect to shift DXPS to an open state. These results offer initial insights to explain the long-lived LThDP intermediate and its activation through disruption of an active site network, which is unique to DXPS. These findings have important implications for DXPS function in bacteria and its development as an antibacterial target.
中文翻译:

活性位点网络的破坏导致抗菌靶标 1-脱氧-d-木酮糖-5-磷酸合酶上的 C2α-乳硫胺二磷酸盐激活
细菌代谢酶 1-脱氧-d-木酮糖-5-磷酸合酶 (DXPS) 催化丙酮酸和d-甘油醛-3-磷酸 ( d -GAP) 依赖二磷酸硫胺素 (ThDP) 形成 DXP。DXP 是一种必需的细菌特异性代谢物,可参与类异戊二烯、磷酸吡哆醛 (PLP) 和 ThDP 的生物合成。DXPS 催化丙酮酸活化,生成 C2α-lactylThDP (LThDP) 加合物,在没有共底物的情况下,该加合物在 DXPS 上以封闭状态长期存在。d -GAP的结合将 DXPS-LThDP 复合物转变为开放状态,这与 LThDP 脱羧一致。这种门控机制在 ThDP 酶学中区分 DXPS。在没有共底物的情况下,LThDP 如何在 DXPS 上持续存在,而其他丙酮酸脱羧酶很容易激活 LThDP 进行脱羧,这是该领域长期存在的问题。我们建议活性位点网络的作用是防止 DXPS 上的 LThDP 激活,直到共底物结合为止。d -GAP的结合与构象转变同时发生,并破坏网络,导致活性位点发生变化,从而促进 LThDP 激活。在这里,我们表明,假定的网络残基以及被认为有助于网络电荷分布的附近残基的替换,可以预见地影响 LThDP 反应性。预计会破坏网络的取代会激活 LThDP 进行脱羧,从而产生 CO 2和乙酸盐。相反,预测增强网络的替代无法激活 LThDP,并且具有将 DXPS 转向关闭状态的效果。LThDP 羧酸盐附近的网络干扰取代也对将 DXPS 转变为开放状态具有显着效果。这些结果为解释长寿命的 LThDP 中间体及其通过破坏活性位点网络而激活提供了初步见解,这是 DXPS 所独有的。这些发现对于 DXPS 在细菌中的功能及其作为抗菌靶点的发展具有重要意义。
更新日期:2024-02-23
中文翻译:

活性位点网络的破坏导致抗菌靶标 1-脱氧-d-木酮糖-5-磷酸合酶上的 C2α-乳硫胺二磷酸盐激活
细菌代谢酶 1-脱氧-d-木酮糖-5-磷酸合酶 (DXPS) 催化丙酮酸和d-甘油醛-3-磷酸 ( d -GAP) 依赖二磷酸硫胺素 (ThDP) 形成 DXP。DXP 是一种必需的细菌特异性代谢物,可参与类异戊二烯、磷酸吡哆醛 (PLP) 和 ThDP 的生物合成。DXPS 催化丙酮酸活化,生成 C2α-lactylThDP (LThDP) 加合物,在没有共底物的情况下,该加合物在 DXPS 上以封闭状态长期存在。d -GAP的结合将 DXPS-LThDP 复合物转变为开放状态,这与 LThDP 脱羧一致。这种门控机制在 ThDP 酶学中区分 DXPS。在没有共底物的情况下,LThDP 如何在 DXPS 上持续存在,而其他丙酮酸脱羧酶很容易激活 LThDP 进行脱羧,这是该领域长期存在的问题。我们建议活性位点网络的作用是防止 DXPS 上的 LThDP 激活,直到共底物结合为止。d -GAP的结合与构象转变同时发生,并破坏网络,导致活性位点发生变化,从而促进 LThDP 激活。在这里,我们表明,假定的网络残基以及被认为有助于网络电荷分布的附近残基的替换,可以预见地影响 LThDP 反应性。预计会破坏网络的取代会激活 LThDP 进行脱羧,从而产生 CO 2和乙酸盐。相反,预测增强网络的替代无法激活 LThDP,并且具有将 DXPS 转向关闭状态的效果。LThDP 羧酸盐附近的网络干扰取代也对将 DXPS 转变为开放状态具有显着效果。这些结果为解释长寿命的 LThDP 中间体及其通过破坏活性位点网络而激活提供了初步见解,这是 DXPS 所独有的。这些发现对于 DXPS 在细菌中的功能及其作为抗菌靶点的发展具有重要意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号