Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-02-23 , DOI: 10.3762/bjoc.20.37 Nahed Ketata , Linhao Liu , Ridha Ben Salem , Henri Doucet
Abstract
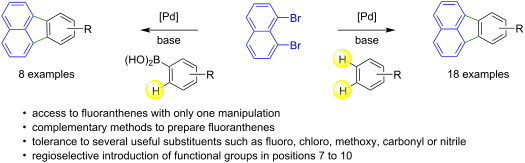
The Pd-catalyzed annulative π-extension of 1,8-dibromonaphthalene for the preparation of fluoranthenes in a single operation has been investigated. With specific arenes such as fluorobenzenes, the Pd-catalyzed double functionalization of C–H bonds yields the desired fluoranthenes. The reaction proceeds via a palladium-catalyzed direct intermolecular arylation, followed by a direct intramolecular arylation step. As the C–H bond activation of several benzene derivatives remains very challenging, the preparation of fluoranthenes from 1,8-dibromonaphthalene via Suzuki coupling followed by intramolecular C–H activation has also been investigated to provide a complementary method. Using the most appropriate synthetic route and substrates, it is possible to introduce the desired functional groups at positions 7–10 on fluoranthenes.
Beilstein J. Org. Chem. 2024, 20, 427–435. doi:10.3762/bjoc.20.37
中文翻译:

单或双 Pd 催化的 C-H 键官能化用于 1,8-二溴萘的环形 π 延伸:一锅法获得荧蒽衍生物
摘要
研究了 Pd 催化的 1,8-二溴萘环 π 延伸在一次操作中制备荧蒽的过程。对于特定的芳烃,例如氟苯,Pd 催化的 C-H 键双官能化产生所需的荧蒽。该反应通过钯催化的直接分子间芳基化进行,然后进行直接分子内芳基化步骤。由于几种苯衍生物的C-H键活化仍然非常具有挑战性,因此还研究了通过Suzuki偶联然后进行分子内C-H活化从1,8-二溴萘制备荧蒽的方法,以提供补充方法。使用最合适的合成路线和底物,可以在荧蒽的 7-10 位引入所需的官能团。

贝尔斯坦 J. 组织。化学。 2024, 20, 427–435。doi:10.3762/bjoc.20.37



























 京公网安备 11010802027423号
京公网安备 11010802027423号