当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Application of Biomaterials in the Development of Hydrogel-Forming Microneedles Integrated with a Cyclodextrin Drug Reservoir for Improved Pharmacokinetic Profiles of Telmisartan
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2024-02-26 , DOI: 10.1021/acsbiomaterials.3c01641 Cindy Kristina Enggi 1 , Sulistiawati Sulistiawati 1 , Achmad Himawan 1, 2 , Muhammad Raihan 1 , Israini Wiyulanda Iskandar 3 , Rizki Rachmad Saputra 4 , Latifah Rahman 1 , Risfah Yulianty 1 , Marianti A. Manggau 1 , Ryan F. Donelly 2 , Muhammad Aswad 1 , Andi Dian Permana 1
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2024-02-26 , DOI: 10.1021/acsbiomaterials.3c01641 Cindy Kristina Enggi 1 , Sulistiawati Sulistiawati 1 , Achmad Himawan 1, 2 , Muhammad Raihan 1 , Israini Wiyulanda Iskandar 3 , Rizki Rachmad Saputra 4 , Latifah Rahman 1 , Risfah Yulianty 1 , Marianti A. Manggau 1 , Ryan F. Donelly 2 , Muhammad Aswad 1 , Andi Dian Permana 1
Affiliation
|
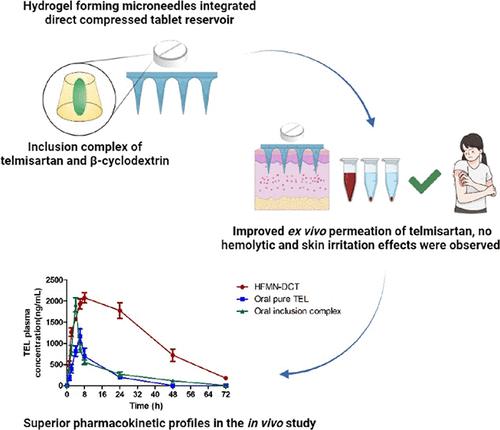
Telmisartan (TEL) is a promising antihypertensive agent among other angiotensin receptor blockers. However, its oral application is limited by its poor water solubility. This study presents the successful utilization of biomaterial-based hydrogel-forming microneedles integrated with a direct compressed tablet reservoir (HFMN-DCT) for the transdermal delivery of telmisartan in the treatment of hypertension. The combination of PVP, PVA, and tartaric acid was used in the HFMN formulation. A range of cross-linking temperatures and times were employed to optimize the characteristics of the HFMN. The HFMN exhibited excellent swelling capacity, mechanical strength, and insertion properties. Additionally, the poorly soluble characteristic of TEL was improved by the inclusion complex formulation with β-cyclodextrin (βCD). Phase solubility analysis showed an Ap-type diagram, indicating a higher-order complex between TEL and βCD, with respect to βCD. A ratio of TEL:βCD of 1:4 mM demonstrates the highest solubility enhancement of TEL. The inclusion complex formation was confirmed by FTIR, XRD, DSC, and molecular docking studies. A significantly higher release of TEL (up to 20-fold) from the inclusion complex was observed in the in vitro release study. Subsequently, a DCT reservoir was developed using various concentrations of sodium starch glycolate. Essentially, both the HFMN and DCT reservoir exhibit hemocompatibility and did not induce any skin irritation. The optimized combination of the HFMN-DCT reservoir showed an ex vivo permeation profile of 83.275 ± 2.405%. Notably, the proposed system showed superior pharmacokinetic profiles in the in vivo investigation using male Wistar rats. Overall, this study highlights the potential of HFMN-DCT reservoir systems as a versatile platform for transdermal drug delivery applications.
中文翻译:

生物材料在开发与环糊精药物储库集成的水凝胶形成微针中的应用,以改善替米沙坦的药代动力学特征
在其他血管紧张素受体阻滞剂中,替米沙坦 (TEL) 是一种很有前途的抗高血压药物。然而,其水溶性较差,限制了其口服应用。本研究成功利用基于生物材料的水凝胶形成微针与直接压缩片剂储库(HFMN-DCT)集成,用于替米沙坦的透皮给药治疗高血压。HFMN 配方中使用了 PVP、PVA 和酒石酸的组合。采用一系列交联温度和时间来优化 HFMN 的特性。HFMN表现出优异的溶胀能力、机械强度和插入性能。此外,TEL 的难溶性特性通过 β-环糊精 (βCD) 的包合物配方得到改善。相溶解度分析显示A p型图,表明相对于βCD,TEL和βCD之间存在高阶复合物。TEL:βCD 的比例为 1:4 mM 表明 TEL 的溶解度增强程度最高。通过 FTIR、XRD、DSC 和分子对接研究证实了包合物的形成。在体外释放研究中观察到包合物中 TEL 的释放显着升高(高达 20 倍)。随后,使用不同浓度的羟基乙酸淀粉钠开发了 DCT 储库。本质上,HFMN 和 DCT 储库均表现出血液相容性,并且不会引起任何皮肤刺激。HFMN-DCT 储库的优化组合显示出 83.275 ± 2.405% 的离体渗透曲线。值得注意的是,所提出的系统在使用雄性 Wistar 大鼠的体内研究中显示出优异的药代动力学特征。总体而言,这项研究强调了 HFMN-DCT 储库系统作为透皮给药应用的多功能平台的潜力。
更新日期:2024-02-26
中文翻译:

生物材料在开发与环糊精药物储库集成的水凝胶形成微针中的应用,以改善替米沙坦的药代动力学特征
在其他血管紧张素受体阻滞剂中,替米沙坦 (TEL) 是一种很有前途的抗高血压药物。然而,其水溶性较差,限制了其口服应用。本研究成功利用基于生物材料的水凝胶形成微针与直接压缩片剂储库(HFMN-DCT)集成,用于替米沙坦的透皮给药治疗高血压。HFMN 配方中使用了 PVP、PVA 和酒石酸的组合。采用一系列交联温度和时间来优化 HFMN 的特性。HFMN表现出优异的溶胀能力、机械强度和插入性能。此外,TEL 的难溶性特性通过 β-环糊精 (βCD) 的包合物配方得到改善。相溶解度分析显示A p型图,表明相对于βCD,TEL和βCD之间存在高阶复合物。TEL:βCD 的比例为 1:4 mM 表明 TEL 的溶解度增强程度最高。通过 FTIR、XRD、DSC 和分子对接研究证实了包合物的形成。在体外释放研究中观察到包合物中 TEL 的释放显着升高(高达 20 倍)。随后,使用不同浓度的羟基乙酸淀粉钠开发了 DCT 储库。本质上,HFMN 和 DCT 储库均表现出血液相容性,并且不会引起任何皮肤刺激。HFMN-DCT 储库的优化组合显示出 83.275 ± 2.405% 的离体渗透曲线。值得注意的是,所提出的系统在使用雄性 Wistar 大鼠的体内研究中显示出优异的药代动力学特征。总体而言,这项研究强调了 HFMN-DCT 储库系统作为透皮给药应用的多功能平台的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号