当前位置:
X-MOL 学术
›
Green Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rapid access to diverse indoles by addition/SNAr with Grignard reagents and 2-fluorophenyl acetonitriles
Green Synthesis and Catalysis Pub Date : 2024-02-24 , DOI: 10.1016/j.gresc.2024.02.004 Yuanyun Gu Gu , Yaxin Feng , Baotong Huang , Yan-En Wang , Yaqi Yuan , Dan Xiong , Yonghong Hu , Xiufang Xu , Patrick J. Walsh , Jianyou Mao
Green Synthesis and Catalysis Pub Date : 2024-02-24 , DOI: 10.1016/j.gresc.2024.02.004 Yuanyun Gu Gu , Yaxin Feng , Baotong Huang , Yan-En Wang , Yaqi Yuan , Dan Xiong , Yonghong Hu , Xiufang Xu , Patrick J. Walsh , Jianyou Mao
|
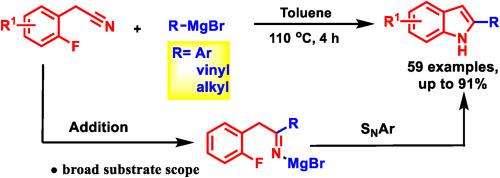
Indoles are essential heterocycles in natural products, biological chemistry, and medicinal chemistry. Efficient approaches to their synthesis, therefore, remain in demand. Herein is reported a novel and scalable method to produce a wide variety of indoles by combining Grignard reagents and 2-fluorobenzyl cyanides (59 examples, 45%–95% yields). The Grignard reagent adds to the nitrile to give a metalated imine that undergoes SAr with unactivated C–F bonds. This strategy installs the R group of RMgX at the indole 2-position, and it is noteworthy that a diverse array of Grignard reagents (aryl, alkyl, vinyl, and cyclopropyl) provide the desired heterocyclic products. The resulting -magnesiated indole can be in situ functionalized at the 3-position with alkyl halides or functionalized on the nitrogen with silyl chlorides. This method enables the synthesis of indoles with functional groups at each position of the indole backbone (C4–C7), providing handles for further functionalization.
中文翻译:

使用格氏试剂和 2-氟苯基乙腈通过加成/SNAr 快速获得多种吲哚
吲哚是天然产物、生物化学和药物化学中重要的杂环化合物。因此,仍然需要有效的合成方法。本文报道了一种新颖且可扩展的方法,通过结合格氏试剂和 2-氟苯甲基氰化物来生产各种吲哚(59 个实例,产率 45%–95%)。格氏试剂添加到腈中,得到金属化亚胺,该亚胺与未活化的 C-F 键发生 SAr。该策略将 RMgX 的 R 基团安装在吲哚 2 位,值得注意的是,多种格氏试剂(芳基、烷基、乙烯基和环丙基)提供了所需的杂环产物。所得的镁化吲哚可以在3-位上用烷基卤化物原位官能化或在氮上用甲硅烷基氯官能化。该方法能够在吲哚主链(C4-C7)的每个位置合成带有官能团的吲哚,为进一步功能化提供了手柄。
更新日期:2024-02-24
中文翻译:

使用格氏试剂和 2-氟苯基乙腈通过加成/SNAr 快速获得多种吲哚
吲哚是天然产物、生物化学和药物化学中重要的杂环化合物。因此,仍然需要有效的合成方法。本文报道了一种新颖且可扩展的方法,通过结合格氏试剂和 2-氟苯甲基氰化物来生产各种吲哚(59 个实例,产率 45%–95%)。格氏试剂添加到腈中,得到金属化亚胺,该亚胺与未活化的 C-F 键发生 SAr。该策略将 RMgX 的 R 基团安装在吲哚 2 位,值得注意的是,多种格氏试剂(芳基、烷基、乙烯基和环丙基)提供了所需的杂环产物。所得的镁化吲哚可以在3-位上用烷基卤化物原位官能化或在氮上用甲硅烷基氯官能化。该方法能够在吲哚主链(C4-C7)的每个位置合成带有官能团的吲哚,为进一步功能化提供了手柄。



























 京公网安备 11010802027423号
京公网安备 11010802027423号