当前位置:
X-MOL 学术
›
Cell Prolif.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
MicroRNA‐29c‐tetrahedral framework nucleic acids: Towards osteogenic differentiation of mesenchymal stem cells and bone regeneration in critical‐sized calvarial defects
Cell Proliferation ( IF 8.5 ) Pub Date : 2024-02-28 , DOI: 10.1111/cpr.13624 Jiafei Sun 1, 2 , Xingyu Chen 1, 2 , Yunfeng Lin 1, 2 , Xiaoxiao Cai 1, 2
Cell Proliferation ( IF 8.5 ) Pub Date : 2024-02-28 , DOI: 10.1111/cpr.13624 Jiafei Sun 1, 2 , Xingyu Chen 1, 2 , Yunfeng Lin 1, 2 , Xiaoxiao Cai 1, 2
Affiliation
|
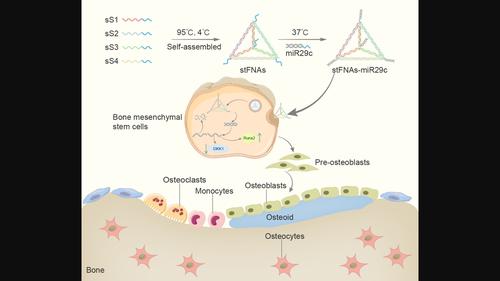
Certain miRNAs, notably miR29c, demonstrate a remarkable capacity to regulate cellular osteogenic differentiation. However, their application in tissue regeneration is hampered by their inherent instability and susceptibility to degradation. In this study, we developed a novel miR29c delivery system utilising tetrahedral framework nucleic acids (tFNAs), aiming to enhance its stability and endocytosis capability, augment the efficacy of miR29c, foster osteogenesis in bone marrow mesenchymal stem cells (BMSCs), and significantly improve the repair of critical‐sized bone defects (CSBDs). We confirmed the successful synthesis and biocompatibility of sticky ends‐modified tFNAs (stFNAs) and miR29c‐modified stFNAs (stFNAs‐miR29c) through polyacrylamide gel electrophoresis, microscopy scanning, a cell counting kit‐8 assay and so on. The mechanism and osteogenesis effects of stFNAs‐miR29c were explored using immunofluorescence staining, western blotting, and reserve transcription quantitative real‐time polymerase chain reaction. Additionally, the impact of stFNAs‐miR29c on CSBD repair was assessed via micro‐CT and histological staining. The nano‐carrier, stFNAs‐miR29c was successfully synthesised and exhibited exemplary biocompatibility. This nano‐nucleic acid material significantly upregulated osteogenic differentiation‐related markers in BMSCs. After 2 months, stFNAs‐miR29c demonstrated significant bone regeneration and reconstruction in CSBDs. Mechanistically, stFNAs‐miR29c enhanced osteogenesis of BMSCs by upregulating the Wnt signalling pathway, contributing to improved bone tissue regeneration. The development of this novel nucleic acid nano‐carrier, stFNAs‐miR29c, presents a potential new avenue for guided bone regeneration and bone tissue engineering research.
中文翻译:

MicroRNA-29c-四面体框架核酸:促进间充质干细胞的成骨分化和临界尺寸颅骨缺损的骨再生
某些 miRNA,特别是 miR29c,表现出调节细胞成骨分化的非凡能力。然而,它们在组织再生中的应用因其固有的不稳定性和易降解性而受到阻碍。在本研究中,我们开发了一种利用四面体框架核酸(tFNA)的新型miR29c递送系统,旨在增强其稳定性和内吞能力,增强miR29c的功效,促进骨髓间充质干细胞(BMSC)的成骨,并显着改善修复临界尺寸骨缺损(CSBD)。我们通过聚丙烯酰胺凝胶电泳、显微镜扫描、细胞计数试剂盒8检测等证实了粘性末端修饰的tFNA(stFNAs)和miR29c修饰的stFNA(stFNAs-miR29c)的成功合成和生物相容性。使用免疫荧光染色、蛋白质印迹和储备转录定量实时聚合酶链反应探讨了 stFNAs-miR29c 的机制和成骨作用。此外,通过显微 CT 和组织学染色评估了 stFNAs-miR29c 对 CSBD 修复的影响。纳米载体 stFNAs-miR29c 已成功合成,并表现出良好的生物相容性。这种纳米核酸材料显着上调 BMSC 中的成骨分化相关标志物。2 个月后,stFNAs-miR29c 在 CSBD 中表现出显着的骨再生和重建。从机制上讲,stFNAs-miR29c 通过上调 Wnt 信号通路增强 BMSC 的成骨作用,有助于改善骨组织再生。这种新型核酸纳米载体stFNAs-miR29c的开发为引导骨再生和骨组织工程研究提供了潜在的新途径。
更新日期:2024-02-28
中文翻译:

MicroRNA-29c-四面体框架核酸:促进间充质干细胞的成骨分化和临界尺寸颅骨缺损的骨再生
某些 miRNA,特别是 miR29c,表现出调节细胞成骨分化的非凡能力。然而,它们在组织再生中的应用因其固有的不稳定性和易降解性而受到阻碍。在本研究中,我们开发了一种利用四面体框架核酸(tFNA)的新型miR29c递送系统,旨在增强其稳定性和内吞能力,增强miR29c的功效,促进骨髓间充质干细胞(BMSC)的成骨,并显着改善修复临界尺寸骨缺损(CSBD)。我们通过聚丙烯酰胺凝胶电泳、显微镜扫描、细胞计数试剂盒8检测等证实了粘性末端修饰的tFNA(stFNAs)和miR29c修饰的stFNA(stFNAs-miR29c)的成功合成和生物相容性。使用免疫荧光染色、蛋白质印迹和储备转录定量实时聚合酶链反应探讨了 stFNAs-miR29c 的机制和成骨作用。此外,通过显微 CT 和组织学染色评估了 stFNAs-miR29c 对 CSBD 修复的影响。纳米载体 stFNAs-miR29c 已成功合成,并表现出良好的生物相容性。这种纳米核酸材料显着上调 BMSC 中的成骨分化相关标志物。2 个月后,stFNAs-miR29c 在 CSBD 中表现出显着的骨再生和重建。从机制上讲,stFNAs-miR29c 通过上调 Wnt 信号通路增强 BMSC 的成骨作用,有助于改善骨组织再生。这种新型核酸纳米载体stFNAs-miR29c的开发为引导骨再生和骨组织工程研究提供了潜在的新途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号