当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Replication Bypass of the N-(2-Deoxy-d-erythro-pentofuranosyl)-urea DNA Lesion by Human DNA Polymerase η
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-27 , DOI: 10.1021/acs.biochem.3c00569 Rachana Tomar 1 , Songlin Li 1 , Martin Egli 2 , Michael P. Stone 1
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-27 , DOI: 10.1021/acs.biochem.3c00569 Rachana Tomar 1 , Songlin Li 1 , Martin Egli 2 , Michael P. Stone 1
Affiliation
|
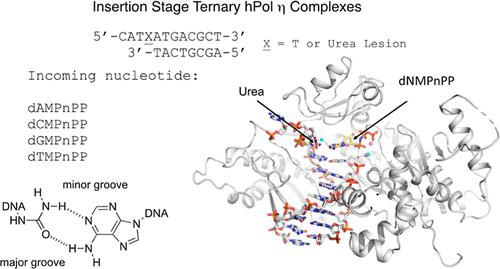
Urea lesions in DNA arise from thymine glycol (Tg) or 8-oxo-dG; their genotoxicity is thought to arise in part due to their potential to accommodate the insertion of all four dNTPs during error-prone replication. Replication bypass with human DNA polymerase η (hPol η) confirmed that all four dNTPs were inserted opposite urea lesions but with purines exhibiting greater incorporation efficiency. X-ray crystal structures of ternary replication bypass complexes in the presence of Mg2+ ions with incoming dNTP analogs dAMPnPP, dCMPnPP, dGMPnPP, and dTMPnPP bound opposite urea lesions (hPol η·DNA·dNMPnPP complexes) revealed all were accommodated by hPol η. In each, the Watson–Crick face of the dNMPnPP was paired with the urea lesion, exploiting the ability of the amine and carbonyl groups of the urea to act as H-bond donors or acceptors, respectively. With incoming dAMPnPP or dGMPnPP, the distance between the imino nitrogen of urea and the N9 atoms of incoming dNMPnPP approximated the canonical distance of 9 Å in B-DNA. With incoming dCMPnPP or dTMPnPP, the corresponding distance of about 7 Å was less ideal. Improved base-stacking interactions were also observed with incoming purines vs pyrimidines. Nevertheless, in each instance, the α-phosphate of incoming dNMPnPPs was close to the 3′-hydroxyl group of the primer terminus, consistent with the catalysis of nucleotidyl transfer and the observation that all four nucleotides could be inserted opposite urea lesions. Preferential insertion of purines by hPol η may explain, in part, why the urea-directed spectrum of mutations arising from Tg vs 8-oxo-dG lesions differs.
中文翻译:

人类 DNA 聚合酶对 N-(2-脱氧-d-赤式-呋喃戊糖基)-尿素 DNA 损伤的复制旁路 η
DNA 中的尿素损伤源自胸腺嘧啶乙二醇 (Tg) 或 8-oxo-dG;人们认为它们的遗传毒性部分是由于它们在容易出错的复制过程中可能容纳所有四种 dNTP 的插入。使用人 DNA 聚合酶 η (hPol η) 的复制旁路证实,所有四种 dNTP 都插入到尿素损伤的对面,但嘌呤表现出更高的掺入效率。在 Mg 2+离子存在下,三元复制旁路复合物的 X 射线晶体结构与传入的 dNTP 类似物 dAMPnPP、dCMPnPP、dGMPnPP 和 dTMPnPP 结合相反的尿素损伤(hPol η·DNA·dNMPnPP 复合物),显示所有这些都由 hPol η 调节。在每个模型中,dNMPnPP 的 Watson-Crick 面与尿素损伤配对,利用尿素的胺基和羰基分别充当氢键供体或受体的能力。对于传入的 dAMPnPP 或 dGMPnPP,尿素的亚氨基氮与传入的 dNMPnPP 的 N9 原子之间的距离接近 B-DNA 中 9 Å 的标准距离。对于传入的 dCMPnPP 或 dTMPnPP,大约 7 Å 的相应距离不太理想。还观察到引入的嘌呤与嘧啶的碱基堆积相互作用得到改善。然而,在每种情况下,传入的 dNMPnPP 的 α-磷酸基团接近引物末端的 3'-羟基,这与核苷酸转移的催化作用以及所有四种核苷酸都可以插入尿素损伤相对的观察结果一致。hPol η 优先插入嘌呤可以部分解释为什么 Tg 与 8-oxo-dG 病变引起的尿素导向突变谱不同。
更新日期:2024-02-27
中文翻译:

人类 DNA 聚合酶对 N-(2-脱氧-d-赤式-呋喃戊糖基)-尿素 DNA 损伤的复制旁路 η
DNA 中的尿素损伤源自胸腺嘧啶乙二醇 (Tg) 或 8-oxo-dG;人们认为它们的遗传毒性部分是由于它们在容易出错的复制过程中可能容纳所有四种 dNTP 的插入。使用人 DNA 聚合酶 η (hPol η) 的复制旁路证实,所有四种 dNTP 都插入到尿素损伤的对面,但嘌呤表现出更高的掺入效率。在 Mg 2+离子存在下,三元复制旁路复合物的 X 射线晶体结构与传入的 dNTP 类似物 dAMPnPP、dCMPnPP、dGMPnPP 和 dTMPnPP 结合相反的尿素损伤(hPol η·DNA·dNMPnPP 复合物),显示所有这些都由 hPol η 调节。在每个模型中,dNMPnPP 的 Watson-Crick 面与尿素损伤配对,利用尿素的胺基和羰基分别充当氢键供体或受体的能力。对于传入的 dAMPnPP 或 dGMPnPP,尿素的亚氨基氮与传入的 dNMPnPP 的 N9 原子之间的距离接近 B-DNA 中 9 Å 的标准距离。对于传入的 dCMPnPP 或 dTMPnPP,大约 7 Å 的相应距离不太理想。还观察到引入的嘌呤与嘧啶的碱基堆积相互作用得到改善。然而,在每种情况下,传入的 dNMPnPP 的 α-磷酸基团接近引物末端的 3'-羟基,这与核苷酸转移的催化作用以及所有四种核苷酸都可以插入尿素损伤相对的观察结果一致。hPol η 优先插入嘌呤可以部分解释为什么 Tg 与 8-oxo-dG 病变引起的尿素导向突变谱不同。



























 京公网安备 11010802027423号
京公网安备 11010802027423号