当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystallographic and Computational Insights into Isoform-Selective Dynamics in Nitric Oxide Synthase
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-28 , DOI: 10.1021/acs.biochem.3c00601 Huiying Li 1 , Christine D. Hardy 1 , Cory T. Reidl 2 , Qing Jing 2 , Fengtian Xue 2 , Maris Cinelli 2 , Richard B. Silverman 2, 3 , Thomas L. Poulos 1
Biochemistry ( IF 2.9 ) Pub Date : 2024-02-28 , DOI: 10.1021/acs.biochem.3c00601 Huiying Li 1 , Christine D. Hardy 1 , Cory T. Reidl 2 , Qing Jing 2 , Fengtian Xue 2 , Maris Cinelli 2 , Richard B. Silverman 2, 3 , Thomas L. Poulos 1
Affiliation
|
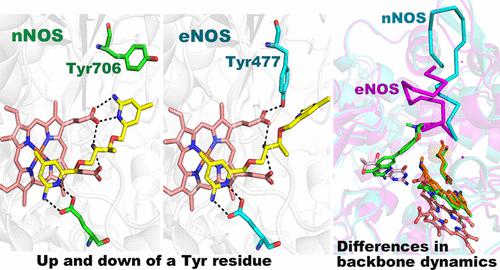
In our efforts to develop inhibitors selective for neuronal nitric oxide synthase (nNOS) over endothelial nitric oxide synthase (eNOS), we found that nNOS can undergo conformational changes in response to inhibitor binding that does not readily occur in eNOS. One change involves movement of a conserved tyrosine, which hydrogen bonds to one of the heme propionates, but in the presence of an inhibitor, changes conformation, enabling part of the inhibitor to hydrogen bond with the heme propionate. This movement does not occur as readily in eNOS and may account for the reason why these inhibitors bind more tightly to nNOS. A second structural change occurs upon the binding of a second inhibitor molecule to nNOS, displacing the pterin cofactor. Binding of this second site inhibitor requires structural changes at the dimer interface, which also occurs more readily in nNOS than in eNOS. Here, we used a combination of crystallography, mutagenesis, and computational methods to better understand the structural basis for these differences in NOS inhibitor binding. Computational results show that a conserved tyrosine near the primary inhibitor binding site is anchored more tightly in eNOS than in nNOS, allowing for less flexibility of this residue. We also find that the inefficiency of eNOS to bind a second inhibitor molecule is likely due to the tighter dimer interface in eNOS compared with nNOS. This study provides a better understanding of how subtle structural differences in NOS isoforms can result in substantial dynamic differences that can be exploited in the development of isoform-selective inhibitors.
中文翻译:

一氧化氮合酶异构体选择性动力学的晶体学和计算见解
在我们努力开发神经元一氧化氮合酶 (nNOS) 而非内皮一氧化氮合酶 (eNOS) 选择性抑制剂的过程中,我们发现 nNOS 可以响应抑制剂结合而发生构象变化,而 eNOS 中不易发生这种变化。一种变化涉及保守酪氨酸的移动,该酪氨酸与一种血红素丙酸酯形成氢键,但在抑制剂存在下,构象发生变化,使部分抑制剂能够与丙酸血红素形成氢键。这种运动在 eNOS 中并不容易发生,这可能是这些抑制剂与 nNOS 结合更紧密的原因。第二个结构变化发生在第二个抑制剂分子与 nNOS 结合时,取代了蝶呤辅助因子。第二个位点抑制剂的结合需要二聚体界面的结构变化,这在 nNOS 中比在 eNOS 中更容易发生。在这里,我们结合使用晶体学、诱变和计算方法来更好地了解 NOS 抑制剂结合差异的结构基础。计算结果表明,主要抑制剂结合位点附近的保守酪氨酸在 eNOS 中比在 nNOS 中锚定得更紧密,因此该残基的灵活性较低。我们还发现,eNOS 结合第二种抑制剂分子的效率低下可能是由于与 nNOS 相比,eNOS 中的二聚体界面更紧密。这项研究让我们更好地了解 NOS 亚型的细微结构差异如何导致显着的动态差异,这些差异可用于开发亚型选择性抑制剂。
更新日期:2024-02-28
中文翻译:

一氧化氮合酶异构体选择性动力学的晶体学和计算见解
在我们努力开发神经元一氧化氮合酶 (nNOS) 而非内皮一氧化氮合酶 (eNOS) 选择性抑制剂的过程中,我们发现 nNOS 可以响应抑制剂结合而发生构象变化,而 eNOS 中不易发生这种变化。一种变化涉及保守酪氨酸的移动,该酪氨酸与一种血红素丙酸酯形成氢键,但在抑制剂存在下,构象发生变化,使部分抑制剂能够与丙酸血红素形成氢键。这种运动在 eNOS 中并不容易发生,这可能是这些抑制剂与 nNOS 结合更紧密的原因。第二个结构变化发生在第二个抑制剂分子与 nNOS 结合时,取代了蝶呤辅助因子。第二个位点抑制剂的结合需要二聚体界面的结构变化,这在 nNOS 中比在 eNOS 中更容易发生。在这里,我们结合使用晶体学、诱变和计算方法来更好地了解 NOS 抑制剂结合差异的结构基础。计算结果表明,主要抑制剂结合位点附近的保守酪氨酸在 eNOS 中比在 nNOS 中锚定得更紧密,因此该残基的灵活性较低。我们还发现,eNOS 结合第二种抑制剂分子的效率低下可能是由于与 nNOS 相比,eNOS 中的二聚体界面更紧密。这项研究让我们更好地了解 NOS 亚型的细微结构差异如何导致显着的动态差异,这些差异可用于开发亚型选择性抑制剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号