当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of 2,2-difluoro-1,3-diketone and 2,2-difluoro-1,3-ketoester derivatives using fluorine gas
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-02-28 , DOI: 10.3762/bjoc.20.41 Alexander S Hampton , David R W Hodgson , Graham McDougald , Linhua Wang , Graham Sandford
中文翻译:

使用氟气合成2,2-二氟-1,3-二酮和2,2-二氟-1,3-酮酯衍生物
更新日期:2024-02-28
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-02-28 , DOI: 10.3762/bjoc.20.41 Alexander S Hampton , David R W Hodgson , Graham McDougald , Linhua Wang , Graham Sandford
Abstract
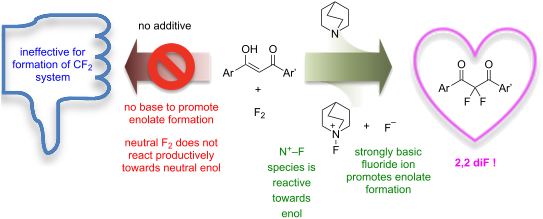
Solutions of 1,3-diketones and 1,3-ketoester derivatives react with fluorine to give the corresponding 2,2-difluoro-1,3-dicarbonyl derivatives in the presence of quinuclidine. Quinuclidine reacts with fluorine in situ to generate a fluoride ion that facilitates limiting enolization processes, and an electrophilic N–F fluorinating agent that is reactive towards neutral enol species.
Beilstein J. Org. Chem. 2024, 20, 460–469. doi:10.3762/bjoc.20.41
中文翻译:

使用氟气合成2,2-二氟-1,3-二酮和2,2-二氟-1,3-酮酯衍生物
摘要
1,3-二酮和1,3-酮酯衍生物的溶液在奎宁环存在下与氟反应生成相应的2,2-二氟-1,3-二羰基衍生物。奎宁环与氟原位反应生成有助于限制烯醇化过程的氟离子,以及对中性烯醇物质具有反应性的亲电子 N-F 氟化剂。

贝尔斯坦 J. 组织。化学。 2024, 20, 460–469。doi:10.3762/bjoc.20.41



























 京公网安备 11010802027423号
京公网安备 11010802027423号