当前位置:
X-MOL 学术
›
Carbon Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unraveling the atomic interdiffusion mechanism of NiFe2O4 oxygen carriers during chemical looping CO2 conversion
Carbon Energy ( IF 20.5 ) Pub Date : 2024-02-27 , DOI: 10.1002/cey2.493 Da Song 1, 2 , Yan Lin 1 , Shiwen Fang 3 , Yang Li 1 , Kun Zhao 1 , Xinfei Chen 1 , Zhen Huang 1, 2 , Fang He 4 , Zengli Zhao 1, 2 , Hongyu Huang 1, 2 , Fanxing Li 5
Carbon Energy ( IF 20.5 ) Pub Date : 2024-02-27 , DOI: 10.1002/cey2.493 Da Song 1, 2 , Yan Lin 1 , Shiwen Fang 3 , Yang Li 1 , Kun Zhao 1 , Xinfei Chen 1 , Zhen Huang 1, 2 , Fang He 4 , Zengli Zhao 1, 2 , Hongyu Huang 1, 2 , Fanxing Li 5
Affiliation
|
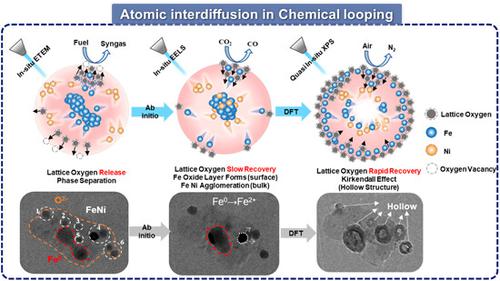
By employing metal oxides as oxygen carriers, chemical looping demonstrates its effectiveness in transferring oxygen between reduction and oxidation environments to partially oxidize fuels into syngas and convert CO2 into CO. Generally, NiFe2O4 oxygen carriers have demonstrated remarkable efficiency in chemical looping CO2 conversion. Nevertheless, the intricate process of atomic migration and evolution within the internal structure of bimetallic oxygen carriers during continuous high-temperature redox cycling remains unclear. Consequently, the lack of a fundamental understanding of the complex ionic migration and oxygen transfer associated with energy conversion processes hampers the design of high-performance oxygen carriers. Thus, in this study, we employed in situ characterization techniques and theoretical calculations to investigate the ion migration behavior and structural evolution in the bulk of NiFe2O4 oxygen carriers during H2 reduction and CO2/lab air oxidation cycles. We discovered that during the H2 reduction step, lattice oxygen rapidly migrates to vacancy layers to replenish consumed active oxygen species, while Ni leaches from the material and migrates to the surface. During the CO2 splitting step, Ni migrates toward the core of the bimetallic oxygen carrier, forming Fe–Ni alloys. During the air oxidation step, Fe–Ni migrates outward, creating a hollow structure owing to the Kirkendall effect triggered by the swift transfer of lattice oxygen. The metal atom migration paths depend on the oxygen transfer rates. These discoveries highlight the significance of regulating the release–recovery rate of lattice oxygen to uphold the structures and reactivity of oxygen carriers. This work offers a comprehensive understanding of the oxidation/reduction-driven atomic interdiffusion behavior of bimetallic oxygen carriers.
中文翻译:

揭示化学链CO2转化过程中NiFe2O4氧载体的原子互扩散机制
通过使用金属氧化物作为氧载体,化学循环展示了其在还原和氧化环境之间转移氧气以将燃料部分氧化成合成气并将CO 2转化为CO的有效性。通常,NiFe 2 O 4氧载体在化学循环CO方面表现出显着的效率2转换。然而,在连续高温氧化还原循环过程中,双金属氧载体内部结构内原子迁移和演化的复杂过程仍不清楚。因此,缺乏对与能量转换过程相关的复杂离子迁移和氧转移的基本了解阻碍了高性能氧载体的设计。因此,在本研究中,我们采用原位表征技术和理论计算来研究NiFe 2 O 4氧载体在H 2还原和CO 2 /实验室空气氧化循环过程中的离子迁移行为和结构演化。我们发现,在H 2还原步骤中,晶格氧快速迁移到空位层以补充消耗的活性氧物种,而Ni从材料中浸出并迁移到表面。在CO 2分解步骤中,Ni 向双金属氧载体的核心迁移,形成 Fe-Ni 合金。在空气氧化步骤中,Fe-Ni 向外迁移,由于晶格氧的快速转移引发的柯肯达尔效应而形成中空结构。金属原子迁移路径取决于氧转移速率。这些发现凸显了调节晶格氧的释放回收率以维持氧载体的结构和反应性的重要性。这项工作提供了对双金属氧载体的氧化/还原驱动的原子互扩散行为的全面理解。
更新日期:2024-02-29
中文翻译:

揭示化学链CO2转化过程中NiFe2O4氧载体的原子互扩散机制
通过使用金属氧化物作为氧载体,化学循环展示了其在还原和氧化环境之间转移氧气以将燃料部分氧化成合成气并将CO 2转化为CO的有效性。通常,NiFe 2 O 4氧载体在化学循环CO方面表现出显着的效率2转换。然而,在连续高温氧化还原循环过程中,双金属氧载体内部结构内原子迁移和演化的复杂过程仍不清楚。因此,缺乏对与能量转换过程相关的复杂离子迁移和氧转移的基本了解阻碍了高性能氧载体的设计。因此,在本研究中,我们采用原位表征技术和理论计算来研究NiFe 2 O 4氧载体在H 2还原和CO 2 /实验室空气氧化循环过程中的离子迁移行为和结构演化。我们发现,在H 2还原步骤中,晶格氧快速迁移到空位层以补充消耗的活性氧物种,而Ni从材料中浸出并迁移到表面。在CO 2分解步骤中,Ni 向双金属氧载体的核心迁移,形成 Fe-Ni 合金。在空气氧化步骤中,Fe-Ni 向外迁移,由于晶格氧的快速转移引发的柯肯达尔效应而形成中空结构。金属原子迁移路径取决于氧转移速率。这些发现凸显了调节晶格氧的释放回收率以维持氧载体的结构和反应性的重要性。这项工作提供了对双金属氧载体的氧化/还原驱动的原子互扩散行为的全面理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号