Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-02-29 , DOI: 10.3762/bjoc.20.43 Ruichen Lan , Brock Yager , Yoonsun Jee , Cynthia S Day , Amanda C Jones
Abstract
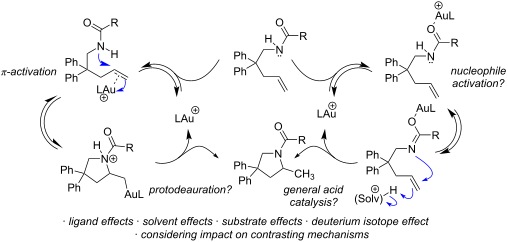
Kinetic studies on the intramolecular hydroamination of protected variants of 2,2-diphenylpent-4-en-1-amine were carried out under a variety of conditions with cationic gold catalysts supported by phosphine ligands. The impact of ligand on gold, protecting group on nitrogen, and solvent and additive on reaction rates was determined. The most effective reactions utilized more Lewis basic ureas, and more electron-withdrawing phosphines. A DCM/alcohol cooperative effect was quantified, and a continuum of isotope effects was measured with low KIE’s in the absence of deuterated alcoholic solvent, increasing to large solvent KIE’s when comparing reactions in pure MeOH to those in pure MeOH-d4. The effects are interpreted both within the context of a classic gold π-activation/protodeauration mechanism and a general acid-catalyzed mechanism without intermediate gold alkyls.
Beilstein J. Org. Chem. 2024, 20, 479–496. doi:10.3762/bjoc.20.43
中文翻译:

金(I)催化分子内烯烃氢胺化中的配体效应、溶剂合作和大动力学溶剂氘同位素效应
摘要
在各种条件下,使用膦配体负载的阳离子金催化剂,对 2,2-二苯基戊-4-en-1-胺受保护变体的分子内氢胺化进行了动力学研究。确定了配体对金、保护基团对氮以及溶剂和添加剂对反应速率的影响。最有效的反应利用更多的路易斯碱性脲和更多的吸电子膦。量化了DCM/醇的协同效应,并在不存在氘代醇溶剂的情况下用低KIE测量了同位素效应的连续体,当比较纯MeOH中的反应与纯MeOH-d 4 中的反应时,增加到大溶剂KIE。这些效应在经典的金 π 激活/原脱气机制和没有中间金烷基的一般酸催化机制的背景下进行解释。

贝尔斯坦 J. 组织。化学。 2024, 20, 479–496。doi:10.3762/bjoc.20.43



























 京公网安备 11010802027423号
京公网安备 11010802027423号