当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
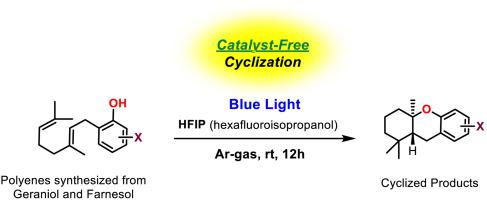
Catalyst-free light-mediated polyene cyclization
Tetrahedron ( IF 2.1 ) Pub Date : 2024-02-27 , DOI: 10.1016/j.tet.2024.133910 Shrijana Bhattarai , Arjun Kafle , Scott T. Handy
Tetrahedron ( IF 2.1 ) Pub Date : 2024-02-27 , DOI: 10.1016/j.tet.2024.133910 Shrijana Bhattarai , Arjun Kafle , Scott T. Handy
|
Polyene cyclization reactions are highly efficient and unique biomimetic transformations that yield complex polycyclic molecules from their acyclic precursors. Various cation-mediated and radical-mediated synthetic methods have been developed to obtain highly diastereoselective cyclizations, along with the use of visible-light in the recent years. In this paper, we report a visible-light-mediated, catalyst-free cascade cyclization of geranyl phenols containing bromo- and chloro-groups at different positions of the phenolic ring. The desired cyclization product was obtained with high stereoselectivity using a blue LED light in hexafluoro-2-propanol without any photocatalyst. The presence of a halogen, and particularly chlorine or bromine is essential for the success of this cyclization, as is the use of blue instead of green LED light. Although the exact reason for this success is unclear, some possible explanations are discussed and further control studies of photoreactions should be a part of any efforts in this area.
中文翻译:

无催化剂光介导多烯环化
多烯环化反应是高效且独特的仿生转化,可从其无环前体产生复杂的多环分子。近年来,随着可见光的使用,已经开发了各种阳离子介导和自由基介导的合成方法来获得高度非对映选择性环化。在本文中,我们报道了在酚环不同位置含有溴基和氯基的香叶基酚的可见光介导、无催化剂级联环化。在不使用任何光催化剂的六氟-2-丙醇中,使用蓝色 LED 灯以高立体选择性获得所需的环化产物。卤素(特别是氯或溴)的存在对于这种环化的成功至关重要,使用蓝色 LED 灯代替绿色 LED 灯也是如此。尽管这一成功的确切原因尚不清楚,但讨论了一些可能的解释,并且光反应的进一步控制研究应该成为该领域任何努力的一部分。
更新日期:2024-02-27
中文翻译:

无催化剂光介导多烯环化
多烯环化反应是高效且独特的仿生转化,可从其无环前体产生复杂的多环分子。近年来,随着可见光的使用,已经开发了各种阳离子介导和自由基介导的合成方法来获得高度非对映选择性环化。在本文中,我们报道了在酚环不同位置含有溴基和氯基的香叶基酚的可见光介导、无催化剂级联环化。在不使用任何光催化剂的六氟-2-丙醇中,使用蓝色 LED 灯以高立体选择性获得所需的环化产物。卤素(特别是氯或溴)的存在对于这种环化的成功至关重要,使用蓝色 LED 灯代替绿色 LED 灯也是如此。尽管这一成功的确切原因尚不清楚,但讨论了一些可能的解释,并且光反应的进一步控制研究应该成为该领域任何努力的一部分。



























 京公网安备 11010802027423号
京公网安备 11010802027423号