当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereoselective Synthesis of 4‐Hydroxy‐1‐Silyl‐1‐Allenylboranes and Access to 2‐Silylethynyl‐1,3‐Diols
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-02-29 , DOI: 10.1002/ajoc.202400042 Ichrak El Haj Brahim 1, 2 , Islem Ishak Dridi 1, 2 , Raoudha Abderrahim 2 , Fabrice Chemla 1 , Franck Ferreira 1 , Olivier Jackowski 1 , Alejandro Pérez‐Luna 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-02-29 , DOI: 10.1002/ajoc.202400042 Ichrak El Haj Brahim 1, 2 , Islem Ishak Dridi 1, 2 , Raoudha Abderrahim 2 , Fabrice Chemla 1 , Franck Ferreira 1 , Olivier Jackowski 1 , Alejandro Pérez‐Luna 1
Affiliation
|
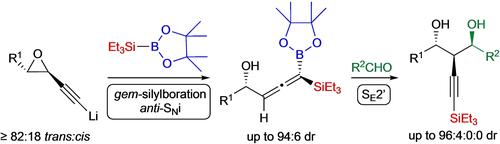
The highly stereoselective gem ‐silylboration of chiral 2‐substituted 1‐ethynylepoxides is reported herein. The reaction involves first the deprotonation of the epoxides in the acetylenic position followed by transmetallation with Et3 SiBpin. The transient silylborane ate‐complexes generated undergo the stereoselective 1,2‐migration of the Et3 Si‐group to the sp‐hybridized terminus carbon of the ethynyl‐moiety in a stereospecific anti ‐addition. The moisture‐sensitive and thermally unstable 4‐hydroxy‐1‐triethylsilyl‐1‐allenyl(pinacolato)boranes thus obtained react with aldehydes through an SE 2’‐mode of addition, affording highly functionalized 2‐triethysilylethynyl‐1,3‐diols with a good diastereoselectivity which is rationalized by a Yamamoto‐Houk type transition state model.
中文翻译:

4-羟基-1-甲硅烷基-1-联烯基硼烷的立体选择性合成及2-甲硅烷基乙炔基-1,3-二醇的制备
高度立体选择性宝石 本文报道了手性2-取代1-乙炔基环氧化物的-甲硅烷基硼化反应。该反应首先涉及环氧化物在乙炔位置的去质子化,然后与 Et 发生金属转移3 西宾。通过 Et 的立体选择性 1,2 迁移而产生瞬时硅基硼烷复合物3 Si-基团与乙炔基部分的 sp-杂化末端碳形成立体特异性反对 -添加。由此获得的对湿气敏感且热不稳定的4-羟基-1-三乙基甲硅烷基-1-联烯基(频哪醇)硼烷通过S与醛反应乙 2'-加成模式,提供高度官能化的2-三乙基甲硅烷基乙炔基-1,3-二醇,具有良好的非对映选择性,这是通过Yamamoto-Houk型过渡态模型合理化的。
更新日期:2024-02-29
中文翻译:

4-羟基-1-甲硅烷基-1-联烯基硼烷的立体选择性合成及2-甲硅烷基乙炔基-1,3-二醇的制备
高度立体选择性



























 京公网安备 11010802027423号
京公网安备 11010802027423号