当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
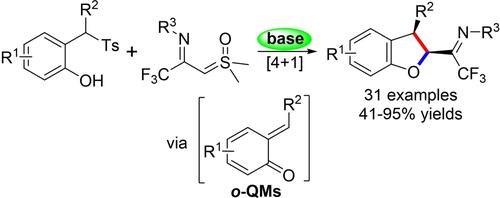
Synthesis of Trifluoromethyl-Containing 2,3-Dihydrobenzofurans via a Formal [4+1] Annulation of In-situ Generated o-Quinone Methides and CF3−Imidoyl Sulfoxonium Ylides
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-02-28 , DOI: 10.1002/ajoc.202400025 Yue Sun 1 , Sihao Ling 1 , Zhengkai Chen 2
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-02-28 , DOI: 10.1002/ajoc.202400025 Yue Sun 1 , Sihao Ling 1 , Zhengkai Chen 2
Affiliation
|
An efficient approach for the synthesis of trifluoroacetimidoyl-substituted dihydrobenzofurans through a metal-free [4+1] annulation reaction of in-situ generated o-QMs with TFISYs has been reported. The protocol enables the formation of various biologically important trifluoromethyl-containing dihydrobenzofurans in up to 95 % yield with good to excellent diastereoselectivities under mild conditions.
中文翻译:

通过原位生成的邻醌甲基化物和 CF3−亚氨基磺叶立德的形式 [4+1] 环化合成含三氟甲基的 2,3-二氢苯并呋喃
据报道,通过原位生成的o -QM与TFISY的无金属[4+1]成环反应合成三氟乙酰亚氨基取代的二氢苯并呋喃的有效方法。该方案能够在温和条件下以高达 95% 的产率形成各种具有重要生物学意义的三氟甲基的二氢苯并呋喃,并具有良好至优异的非对映选择性。
更新日期:2024-02-28
中文翻译:

通过原位生成的邻醌甲基化物和 CF3−亚氨基磺叶立德的形式 [4+1] 环化合成含三氟甲基的 2,3-二氢苯并呋喃
据报道,通过原位生成的o -QM与TFISY的无金属[4+1]成环反应合成三氟乙酰亚氨基取代的二氢苯并呋喃的有效方法。该方案能够在温和条件下以高达 95% 的产率形成各种具有重要生物学意义的三氟甲基的二氢苯并呋喃,并具有良好至优异的非对映选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号