当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The chemistry of disorazoles and structure-activity relationships: An update
Tetrahedron ( IF 2.1 ) Pub Date : 2024-02-29 , DOI: 10.1016/j.tet.2024.133908 Christian P. Bold , Karl-Heinz Altmann
Tetrahedron ( IF 2.1 ) Pub Date : 2024-02-29 , DOI: 10.1016/j.tet.2024.133908 Christian P. Bold , Karl-Heinz Altmann
|
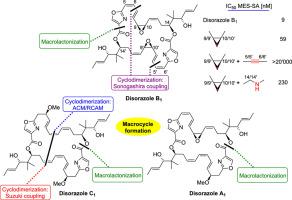
The disorazoles are a family of 26- to 32-membered macrodiolides of mixed non-ribosomal peptide and polyketide origin that show exceptional cytotoxic activity. Of the 39 different disorazoles that have been isolated so far, total syntheses have been described for three family members, namely the non-symmetrical disorazole A and the symmetrical disorazoles B and C. With the exception of the first total synthesis of disorazole C, these syntheses were all reported within the last decade. Different approaches have been followed to meet the challenge of establishing the 30-membered macrodiolide ring in these natural products, including single-step macrolactonization or cyclodimerization sequential inter-/intramolecular Suzuki coupling, ACM/RCAM, or inter-/intramolecular Sonogashira coupling. In this report, we review the total syntheses of disorazoles that have been developed over the last decade. In addition, we also summarize the synthetic and SAR work that has been performed on non-natural disorazole congeners, which built on the chemistry developed in the course of the total synthesis work.
中文翻译:

双索唑的化学性质和构效关系:更新
地索拉唑是混合非核糖体肽和聚酮化合物来源的 26 至 32 元大环二内酯家族,具有出色的细胞毒活性。迄今为止,已分离出 39 种不同的双索唑,其中三个家族成员的全合成已被描述,即非对称双索唑 A 和对称双索唑 B 和 C。除了首次全合成双索唑 C 外,这些合成方法均是在过去十年内报道的。为了应对在这些天然产物中建立 30 元大二内酯环的挑战,人们采用了不同的方法,包括单步大内酯化或环二聚化顺序分子间/内 Suzuki 偶联、ACM/RCAM 或分子间/分子内 Sonogashira 偶联。在本报告中,我们回顾了过去十年中开发的双索唑的全合成。此外,我们还总结了对非天然地索拉唑同系物进行的合成和SAR工作,这些工作建立在全合成工作过程中开发的化学基础上。
更新日期:2024-02-29
中文翻译:

双索唑的化学性质和构效关系:更新
地索拉唑是混合非核糖体肽和聚酮化合物来源的 26 至 32 元大环二内酯家族,具有出色的细胞毒活性。迄今为止,已分离出 39 种不同的双索唑,其中三个家族成员的全合成已被描述,即非对称双索唑 A 和对称双索唑 B 和 C。除了首次全合成双索唑 C 外,这些合成方法均是在过去十年内报道的。为了应对在这些天然产物中建立 30 元大二内酯环的挑战,人们采用了不同的方法,包括单步大内酯化或环二聚化顺序分子间/内 Suzuki 偶联、ACM/RCAM 或分子间/分子内 Sonogashira 偶联。在本报告中,我们回顾了过去十年中开发的双索唑的全合成。此外,我们还总结了对非天然地索拉唑同系物进行的合成和SAR工作,这些工作建立在全合成工作过程中开发的化学基础上。



























 京公网安备 11010802027423号
京公网安备 11010802027423号