当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Progress in the synthesis of polyoxamic acids
Tetrahedron ( IF 2.1 ) Pub Date : 2024-02-28 , DOI: 10.1016/j.tet.2024.133913 Bhagirath Limbani , Smritilekha Bera , Dhananjoy Mondal
Tetrahedron ( IF 2.1 ) Pub Date : 2024-02-28 , DOI: 10.1016/j.tet.2024.133913 Bhagirath Limbani , Smritilekha Bera , Dhananjoy Mondal
|
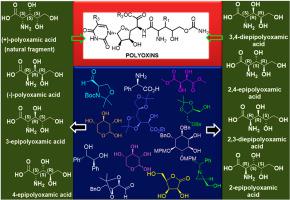
Polyoxamic acid is a crucial part of polyoxins, which are a family of naturally occurring nucleoside peptide antibiotics and, inhibits chitin synthase from Candida albicans as potential antifungal agents. The biological activity of polyoxins is closely linked to the presence of polyoxamic acid moiety in the framework of the molecule. With three contiguous chiral centers, polyoxamic acid can exist in eight stereoisomers. Due to its biological significance and prevalence in the structure of natural products, the synthesis of polyoxamic acid and its analogues have been extensively investigated. Numerous methods, including those using chiral ligands, chiral auxiliaries, and carbohydrate chemistry, have been explored. Total synthesis strategies for polyoxamic acid have evolved, incorporating catalytic Sharpless asymmetric dihydroxylation, epoxidation, aldol condensation, and Overman [3,3]-sigmatropic rearrangement, among others. This review comprehensively focused on the various synthetic approaches employed for natural polyoxamic acid and its stereoisomers.
中文翻译:

聚草酸的合成研究进展
聚乙酰胺酸是多抗素的重要组成部分,多抗素是一类天然存在的核苷肽抗生素,可抑制白色念珠菌的几丁质合酶,作为潜在的抗真菌剂。多抗素的生物活性与分子框架中聚草酸部分的存在密切相关。聚肟酸具有三个连续的手性中心,可以存在八种立体异构体。由于其生物学意义和在天然产物结构中的普遍性,聚草酸及其类似物的合成已被广泛研究。人们已经探索了许多方法,包括使用手性配体、手性助剂和碳水化合物化学的方法。聚草酸的全合成策略已经发展,包括催化 Sharpless 不对称二羟基化、环氧化、羟醛缩合和 Overman [3,3]-σ 重排等。本综述全面关注天然聚肟酸及其立体异构体的各种合成方法。
更新日期:2024-02-28
中文翻译:

聚草酸的合成研究进展
聚乙酰胺酸是多抗素的重要组成部分,多抗素是一类天然存在的核苷肽抗生素,可抑制白色念珠菌的几丁质合酶,作为潜在的抗真菌剂。多抗素的生物活性与分子框架中聚草酸部分的存在密切相关。聚肟酸具有三个连续的手性中心,可以存在八种立体异构体。由于其生物学意义和在天然产物结构中的普遍性,聚草酸及其类似物的合成已被广泛研究。人们已经探索了许多方法,包括使用手性配体、手性助剂和碳水化合物化学的方法。聚草酸的全合成策略已经发展,包括催化 Sharpless 不对称二羟基化、环氧化、羟醛缩合和 Overman [3,3]-σ 重排等。本综述全面关注天然聚肟酸及其立体异构体的各种合成方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号