当前位置:
X-MOL 学术
›
Nano Today
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Procoagulant CaCO3-embedded embolic microspheres can potentiate transcatheter arterial embolization of hepatocellular carcinoma
Nano Today ( IF 17.4 ) Pub Date : 2024-02-28 , DOI: 10.1016/j.nantod.2024.102216 Ziliang Dong , Lei Zhang , Dongxu Zhao , Chunjie Wang , Yu Hao , Quguang Li , Yunyun Zhang , Zhijuan Yang , Caifang Ni , Zhuang Liu , Liangzhu Feng
Nano Today ( IF 17.4 ) Pub Date : 2024-02-28 , DOI: 10.1016/j.nantod.2024.102216 Ziliang Dong , Lei Zhang , Dongxu Zhao , Chunjie Wang , Yu Hao , Quguang Li , Yunyun Zhang , Zhijuan Yang , Caifang Ni , Zhuang Liu , Liangzhu Feng
|
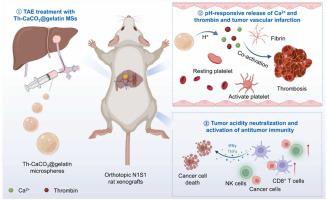
Transcatheter arterial embolization (TAE) represents a preferred therapeutic modality with minimal invasiveness for unresectable intermediate and advanced hepatocellular carcinoma (HCC) patients. However, its therapeutic outcome is restricted by incomplete occlusion of the whole tumor vasculature and exacerbated tumor immunosuppression due to the lack of ideal embolic agents. Herein, procoagulant embolic microspheres with tunable sizes are prepared by loading thrombin into calcium carbonate (CaCO) nanoparticle-embedded gelatin microspheres (Th-CaCO@gelatin MSs) via a microfluidic process. In this system, the yielded Th-CaCO@gelatin MSs are capable of occluding main tumor-feeding blood vessels, while the released thrombin and Ca triggered by the acidic tumor microenvironment can synergistically promote the formation of intravascular blood clots to occlude tumor capillaries. Meanwhile, the embedded CaCO nanoparticles can work as proton sponges to neutralize tumor acidity and thus effectively reverse tumor immunosuppression. Resultantly, TAE treatment with Th-CaCO@gelatin MSs exhibits the most effective tumor suppression efficacy on orthotopic N1S1 HCC rat xenografts without causing obvious toxic effects. Therefore, this study highlights that our biocompatible embolic microspheres are capable of potentiating conventional TAE treatment by promoting pH-responsive tumor vascular infarction and neutralizing the acidic tumor microenvironment, with great potential in clinical translation.
中文翻译:

促凝 CaCO3 包埋栓塞微球可增强肝细胞癌的经导管动脉栓塞
经导管动脉栓塞(TAE)是不可切除的中晚期肝细胞癌(HCC)患者的首选治疗方式,具有微创性。然而,由于缺乏理想的栓塞剂,其治疗效果受到整个肿瘤脉管系统的不完全闭塞和肿瘤免疫抑制加剧的限制。在此,通过微流体过程将凝血酶负载到碳酸钙(CaCO)纳米颗粒嵌入的明胶微球(Th-CaCO@明胶MS)中,制备了尺寸可调的促凝栓塞微球。在该系统中,产生的Th-CaCO@明胶MS能够闭塞主要的肿瘤供血血管,而酸性肿瘤微环境触发释放的凝血酶和Ca可以协同促进血管内血栓的形成以闭塞肿瘤毛细血管。同时,嵌入的CaCO纳米粒子可以作为质子海绵来中和肿瘤的酸度,从而有效逆转肿瘤的免疫抑制。因此,Th-CaCO@明胶 MS 的 TAE 治疗对原位 N1S1 HCC 大鼠异种移植物表现出最有效的肿瘤抑制功效,且不会引起明显的毒性作用。因此,本研究强调,我们的生物相容性栓塞微球能够通过促进pH响应性肿瘤血管梗塞和中和酸性肿瘤微环境来增强常规TAE治疗,在临床转化方面具有巨大潜力。
更新日期:2024-02-28
中文翻译:

促凝 CaCO3 包埋栓塞微球可增强肝细胞癌的经导管动脉栓塞
经导管动脉栓塞(TAE)是不可切除的中晚期肝细胞癌(HCC)患者的首选治疗方式,具有微创性。然而,由于缺乏理想的栓塞剂,其治疗效果受到整个肿瘤脉管系统的不完全闭塞和肿瘤免疫抑制加剧的限制。在此,通过微流体过程将凝血酶负载到碳酸钙(CaCO)纳米颗粒嵌入的明胶微球(Th-CaCO@明胶MS)中,制备了尺寸可调的促凝栓塞微球。在该系统中,产生的Th-CaCO@明胶MS能够闭塞主要的肿瘤供血血管,而酸性肿瘤微环境触发释放的凝血酶和Ca可以协同促进血管内血栓的形成以闭塞肿瘤毛细血管。同时,嵌入的CaCO纳米粒子可以作为质子海绵来中和肿瘤的酸度,从而有效逆转肿瘤的免疫抑制。因此,Th-CaCO@明胶 MS 的 TAE 治疗对原位 N1S1 HCC 大鼠异种移植物表现出最有效的肿瘤抑制功效,且不会引起明显的毒性作用。因此,本研究强调,我们的生物相容性栓塞微球能够通过促进pH响应性肿瘤血管梗塞和中和酸性肿瘤微环境来增强常规TAE治疗,在临床转化方面具有巨大潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号