当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Measurement and Thermodynamic Modeling of Oxaprozin Solubility in Polymers and Mixed Solutions
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-05 , DOI: 10.1021/acs.jced.3c00564 Haomin Wu 1 , YingYing Jiang 1 , Chen Shen 1 , Yuanhui Ji 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-05 , DOI: 10.1021/acs.jced.3c00564 Haomin Wu 1 , YingYing Jiang 1 , Chen Shen 1 , Yuanhui Ji 1
Affiliation
|
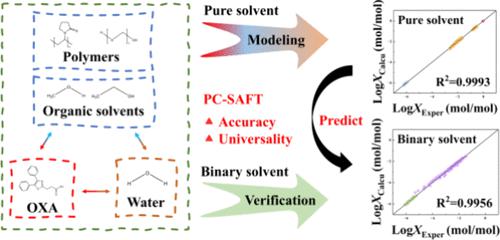
Measuring and modeling the solubility of drugs in different solvent systems is helpful to guide the selection of appropriate solvents at various stages of drug formulation development. In this work, the gravimetrical method was used to determine the solubility data of oxaprozin (OXA) in different compositions of water/organic solvents (methanol and ethanol) binary mixtures within the range of 293.15 to 333.15 K. Subsequently, the differential scanning calorimetry method was used to measure the solubility of the drug in polymers (Polyethylene Glycol 6000 and Polyvinylpyrrolidone K30) at above 400 K. Then, the solubility of OXA in ultrapure water and polymer aqueous solution was acquired by UV spectrophotometry or HPLC within a temperature range of 303.15 to 323.15 K. Finally, the experimental values were compared with the calculated values from the Perturbed-Chain Statistical Associating Fluid Theory (PC-SAFT) to investigate the prediction accuracy of this model in different complex mixed solvent systems. The average relative deviations (ARD) were used to evaluate the model performance of PC-SAFT. Furthermore, PC-SAFT combined with solid–liquid equilibrium theory not only modeled the phase behavior between pure or mixed solvents and drugs independent of the molecular weight of the solvent but also did not require any experimental data or model parameters from the ternary system to predict the phase behavior of OXA in binary solvents. The results of this work illustrate that PC-SAFT is a beneficial model in drug development.
中文翻译:

奥沙普秦在聚合物和混合溶液中溶解度的测量和热力学建模
测量和建模药物在不同溶剂系统中的溶解度有助于指导在药物制剂开发的各个阶段选择合适的溶剂。本工作采用重量法测定了奥沙普秦(OXA)在不同组成的水/有机溶剂(甲醇和乙醇)二元混合物中在293.15至333.15 K范围内的溶解度数据。随后,差示扫描量热法在400 K以上测量药物在聚合物(聚乙二醇6000和聚乙烯吡咯烷酮K30)中的溶解度。然后,在303.15温度范围内通过紫外分光光度法或HPLC获得OXA在超纯水和聚合物水溶液中的溶解度。最后,将实验值与扰动链统计缔合流体理论(PC-SAFT)的计算值进行比较,以研究该模型在不同复杂混合溶剂体系中的预测精度。平均相对偏差(ARD)用于评估PC-SAFT的模型性能。此外,PC-SAFT与固液平衡理论相结合,不仅模拟了纯溶剂或混合溶剂与药物之间的相行为,与溶剂的分子量无关,而且不需要三元体系的任何实验数据或模型参数来预测OXA 在二元溶剂中的相行为。这项工作的结果表明 PC-SAFT 是药物开发中的一种有益模型。
更新日期:2024-03-05
中文翻译:

奥沙普秦在聚合物和混合溶液中溶解度的测量和热力学建模
测量和建模药物在不同溶剂系统中的溶解度有助于指导在药物制剂开发的各个阶段选择合适的溶剂。本工作采用重量法测定了奥沙普秦(OXA)在不同组成的水/有机溶剂(甲醇和乙醇)二元混合物中在293.15至333.15 K范围内的溶解度数据。随后,差示扫描量热法在400 K以上测量药物在聚合物(聚乙二醇6000和聚乙烯吡咯烷酮K30)中的溶解度。然后,在303.15温度范围内通过紫外分光光度法或HPLC获得OXA在超纯水和聚合物水溶液中的溶解度。最后,将实验值与扰动链统计缔合流体理论(PC-SAFT)的计算值进行比较,以研究该模型在不同复杂混合溶剂体系中的预测精度。平均相对偏差(ARD)用于评估PC-SAFT的模型性能。此外,PC-SAFT与固液平衡理论相结合,不仅模拟了纯溶剂或混合溶剂与药物之间的相行为,与溶剂的分子量无关,而且不需要三元体系的任何实验数据或模型参数来预测OXA 在二元溶剂中的相行为。这项工作的结果表明 PC-SAFT 是药物开发中的一种有益模型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号