当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
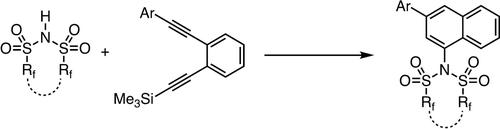
Synthesis of 1-amino-3-aryl Naphthalenes from Bis(perfluoroalkanesulfonyl)imide with 1,2-diethynylbenzenes
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-03-04 , DOI: 10.1002/ajoc.202400035 Takuji Kawamoto 1 , Tetsushi Yamasaki 2 , Tsubasa Ikazaki 2 , Hiroshi Matsubara 3 , Akio Kamimura 2
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-03-04 , DOI: 10.1002/ajoc.202400035 Takuji Kawamoto 1 , Tetsushi Yamasaki 2 , Tsubasa Ikazaki 2 , Hiroshi Matsubara 3 , Akio Kamimura 2
Affiliation
|
Although the synthesis of vinyl triflates has a long history, the synthesis of vinyl bis(perfluoroalkanesulfonyl)imides has not been reported yet. Here, we report a simple synthetic route to vinyl bis(perfluoroalkanesulfonyl)imides that does not require any additives. The stereoselectivity originates from the steric hindrance between the aryl group and the imide. The reaction proceeds chemoselectively, and subsequent cyclization provides the 1-amino-3-aryl naphthalene dewrivatives.
中文翻译:

双(全氟烷磺酰基)亚胺与1,2-二乙炔基苯合成1-氨基-3-芳基萘
尽管乙烯基三氟甲磺酸酯的合成已有悠久的历史,但乙烯基双(全氟烷磺酰基)亚胺的合成尚未见报道。在这里,我们报告了一种不需要任何添加剂的乙烯基双(全氟烷磺酰基)亚胺的简单合成路线。立体选择性源于芳基和酰亚胺之间的空间位阻。该反应以化学选择性进行,随后环化得到1-氨基-3-芳基萘衍生物。
更新日期:2024-03-04
中文翻译:

双(全氟烷磺酰基)亚胺与1,2-二乙炔基苯合成1-氨基-3-芳基萘
尽管乙烯基三氟甲磺酸酯的合成已有悠久的历史,但乙烯基双(全氟烷磺酰基)亚胺的合成尚未见报道。在这里,我们报告了一种不需要任何添加剂的乙烯基双(全氟烷磺酰基)亚胺的简单合成路线。立体选择性源于芳基和酰亚胺之间的空间位阻。该反应以化学选择性进行,随后环化得到1-氨基-3-芳基萘衍生物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号